- About
-
Services
-
Offerings
- Offerings
- ADME and Bioanalytical Sciences
- Analytical Chemistry
- Assay Development
- Biochemical Assays
- Biophysical Assays
- Cell Based Assays
- Computational Chemistry
- Fragment and Compound Screening
- Integrated Drug Discovery Services
- Medicinal Chemistry
- Project Management and Consultancy Services
- Protein Expression and Purification Services
- Structural Biology
- Synthetic Chemistry
- Virtual screening
-
Research Phases
- Research Phases
- Hit Identification
- Hit to Lead
- Lead Optimisation
- Therapeutic Areas
- Target Classes
-
Approaches & Techniques
- Approaches & Techniques
- CDH (Target Gene Fragmentation)
- Cryogenic Electron Microscopy (Cryo-EM)
- Differential Scanning Fluorimetry (DSF) and nanoDSF Services
- Direct-to-Biology (D2B)
- Dynamic Light Scattering (DLS)
- eProtein Discovery
- Flow Cytometry
- Fragment Based Drug Discovery (FBDD)
- FragmentBuilder
- Grating-Coupled Interferometry
- High Throughput Screening
- Isothermal Titration Calorimetry (ITC)
- LeadBuilder
- Photoaffinity Labelling
- PoLiPa (Membrane Protein Solubilisation)
- Spectral Shift and MST Services
- Structure Based Drug Design (SBDD)
- Surface Plasmon Resonance (SPR)
- X-ray Crystallography
-
Offerings
- Library
- News & Events
- Careers
VEGF/NRP1: PPI inhibitors for the treatment of solid tumours
Domainex helped develop what are believed to be the first published small molecule antagonists with nanomolar potencies for the neuropilin 1 (NRP1) receptor.
Challenge
NRP1 is a receptor for vascular endothelial growth factor A165 (VEGF-A165) and the neuronal guidance molecule semaphorin 3A (SEMA3A)1, with key roles in vascular and neuronal development. In endothelial cells, NRP1 enhances the biological signals of VEGF-A mediated by binding to its receptor vascular endothelial growth factor 2 (VEGFR2); see Figure 1.
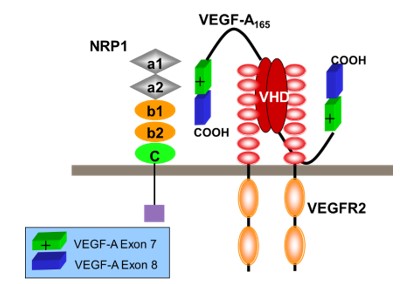
Figure 1: Model for binding of VEGF-A165 to NRP1. NRP1 has a large extracellular (Ex) domain comprising tandem a1/a2, b1/2, and a c domain, a single membrane-spanning domain, and a small cytosolic domain. The VEGF-A165 C-terminal domain encoded by exons 7 and 8 (green and blue respectively) binds to the extracellular NRP1 b1 domain. Concomitant binding of the VEGF homology domain of VEGF-A165 (solid red ovals) to VEGFR2 results in formation of a receptor complex of NRP1 with VEGF-A165 and VEGFR2 resulting in enhanced intracellular signalling, essential for optimal migration and angiogenesis in development and in tumours.
NRP1 has been implicated in tumour growth and angiogenesis. Pan et al.2 have shown that inhibition by a blocking antibody that prevents VEGF-A binding to NRP1 enhances the anti-tumour effects of the inhibitory anti-VEGF-A antibody, bevacizumab (Avastin®) in mouse xenograft models. It is therefore believed that a small molecule inhibitor of NRP1 function would be desirable, but inhibitors of protein-protein interactions (PPIs) are not trivial to develop.
Solution
Domainex helped develop inhibitors of this PPI, using a peptidomimetic approach. The team followed a rational design strategy:
- Identify the minimal binding epitope of the natural ligand, VEGF-A165
- Characterise key binding interactions
- Design peptidomimetics
Identifying the minimal binding epitope
Starting with the full Exon7-8 sequence, by making a series of truncated molecules we discovered that the C-terminal peptide Ac-RXDKPRR-OH (where X=2-aminobutyric acid) of VEGF-A retained significant affinity for NRP1. Substitution of the nonessential penultimate arginine to alanine and N-terminal truncation led to the discovery that peptide H-KPAR-OH, was a significantly active inhibitor with a minimal peptide sequence. Our medicinal chemists performed some SAR studies around this template (Table 1) and this information was used as the starting point for the subsequent design of peptidomimetics.
| Peptide Sequence | Inhibition of 125I-VEGF binding to PAE/NRP1† cells | |
|---|---|---|
| % at 100 μM | IC50 μM | |
| Ac-RXbDKPAR-OH* | 71 | 18 |
| H-kPAR-OH | 83 | 30 |
| H-kPAR-OH | 51 | |
| H-KPaR-OH | 36 | |
| H-KPPR-OH | 96 | 14 |
| H-KPFR-OH | 63 | |
| H-KPAr-OH | 22 | |
| H-KPAK-OH | 22 | |
| H-KPAQ-OH | 19 | |
* X=2-aminobutyric acid
†PAE/NRP1 = Porcine aortal endothelial cells expressing NRP1
Characterising key binding interactions
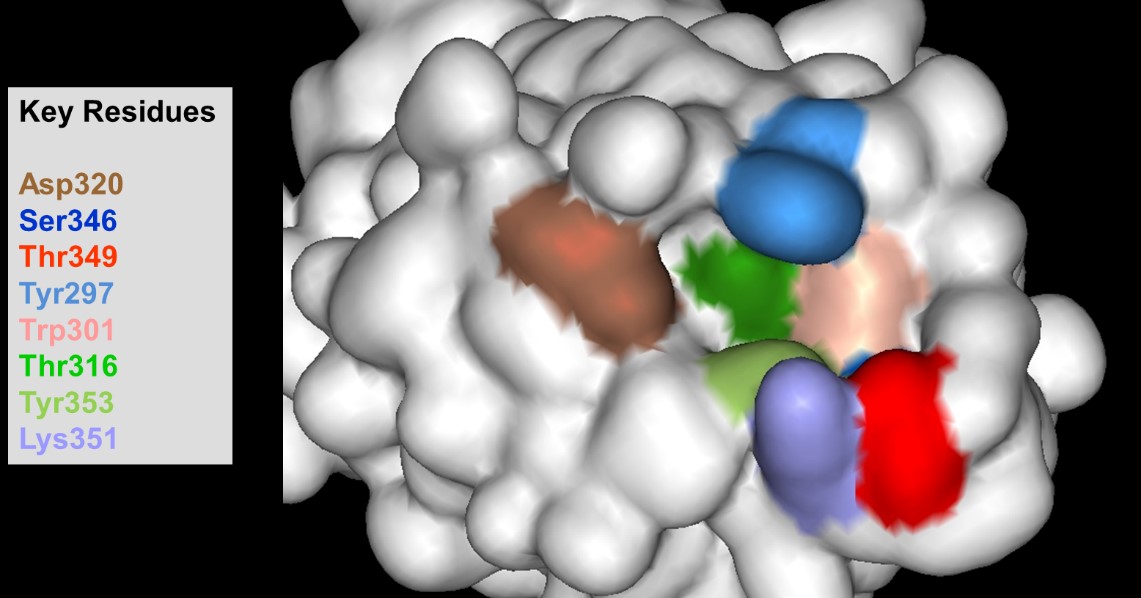
Mutagenesis studies localised VEGF-A binding to the NRP1 b1 domain and a binding pocket was identified. Figure 2 shows the key residues – one of which was Asp320 – which formed a guanidine-binding pocket.
Designing peptidomimetics
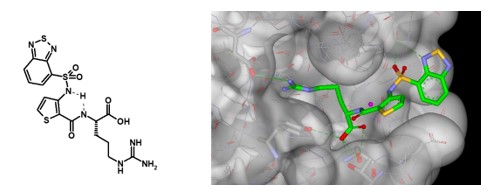
We undertook a programme of medicinal chemistry, applying a peptidomimetic approach, and this work resulted in the design and synthesis of EG00229 (Figure 3).
EG00229 inhibited VEGF-A165 binding to:
- PAE/NRP1 cells
- lung carcinoma A549 cells
- prostate carcinoma DU145 cells (which express NRP1, but not VEGFR1 or VEGFR2)
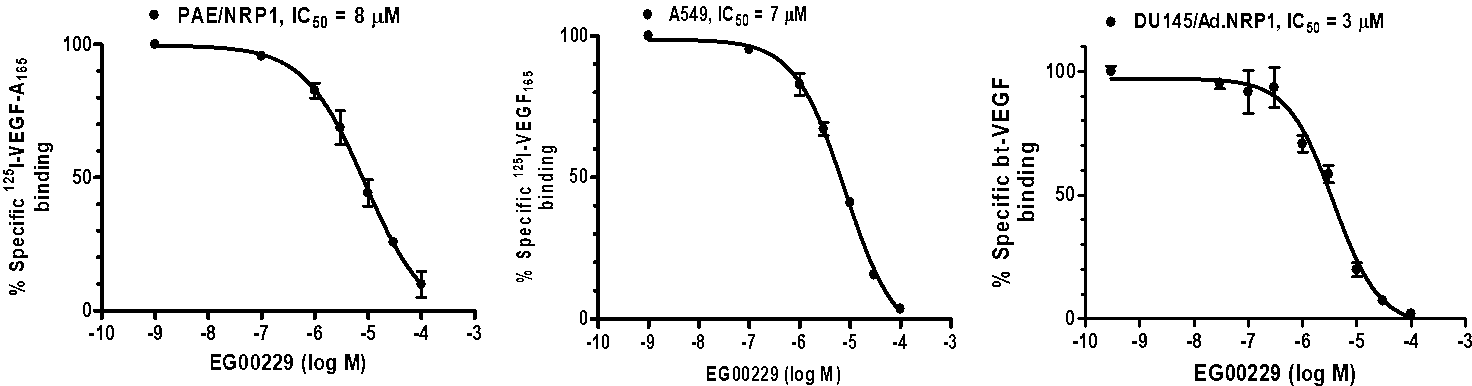
Figure 4: EG00229 inhibits the binding of VEGF-A165
EG00229 also enhanced the potency of cytotoxic drugs, including 5-fluorouracil (5-FU) and paclitaxel, to carcinoma cells (Figure 5).
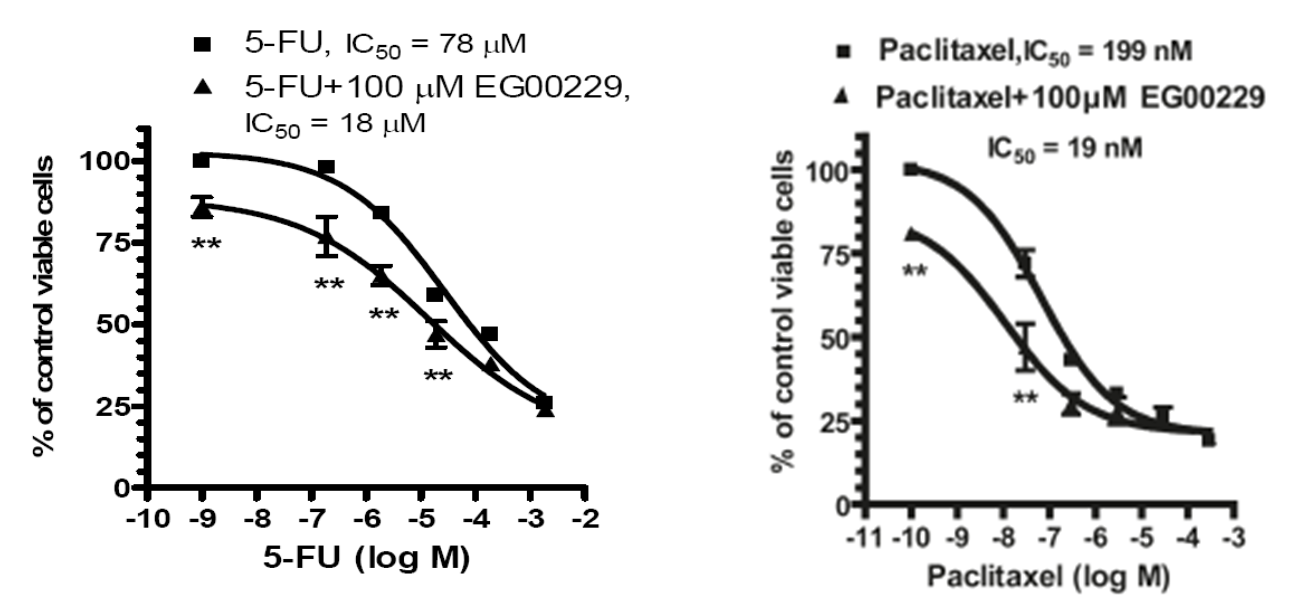
Conclusions
NRP1 blockers have the potential to target angiogenesis, tumour growth and metastasis and may provide a valuable alternative to targeted cancer therapy, particularly relevant in the light of concern over findings showing that anti-VEGF agents may stimulate metastasis.3 We designed and synthesised EG00229, the first small-molecule ligand of the VEGF-A165 receptor NRP1. EG00229 and its analogues made excellent tools for the study of NRP1 biology and provided a point of departure for the development of more potent anti-NRP1 drugs.4
This work resulted in two publications.1,5
Domainex Expertise
• Medicinal and Synthetic Chemistry • Structure-based Drug Design (SBDD) • Hit-to-lead
• Peptidomimetic • Protein-protein Interaction Drug Discovery • Oncology
References
1. N-Terminal Modification of VEGF-A C Terminus-Derived Peptides Delineates Structural Features Involved in Neuropilin-1 Binding and Functional Activity. Haiyan Jia, Rehan Aqil, Lili Cheng, Chris Chapman, Shaheda Shaikh, Ashley Jarvis, A. W. Edith Chan, Basil Hartzoulakis, Ian M. Evans, Antonina Frolov, John Martin, Paul Frankel, Snezana Djordevic, Ian C. Zachary and David L. Selwood., ChemBioChem., 2014, 15, 8, 1161-1170.
2. Blocking Neuropilin-1 Function Has an Additive Effect with Anti-VEGF to Inhibit Tumor Growth. Qi Pan, Yvan Chanthery, Wei-Ching Liang, Scott Stawicki, Judy Mak, Nisha Rathore, Raymond K. Tong, Joe Kowalski, Sharon Fong Yee, Glenn Pacheco, Sarajane Ross, Zhiyong Cheng, Jennifer Le Couter, Greg Plowman, Franklin Peale, Alexander W. Koch, Yan Wu, Anil Bagri, Marc Tessier-Lavigne and Ryan J. Watts. Cancer Cell, 2007, 11, 53–67.
3. Anti-VEGF therapy as adjuvant therapy: clouds on the horizon? Schneider, B. P.; Sledge, G. W., Jr. Breast Cancer Res. 2009, 11, 303.
4. Small Molecule Neuropilin-1 Antagonists Combine Antiangiogenic and Antitumor Activity with Immune Modulation through Reduction of Transforming Growth Factor Beta (TGFβ) Production in Regulatory T-Cells. Jonathan Powell, Filipa Mota, David Steadman, Christelle Soudy, Jeremy T. Miyauchi, Stuart Crosby, Ashley Jarvis, Tifelle Reisinger, Natalie Winfield, Graham Evans, Aled Finniear, Tamas Yelland, Yi-Tai Chou, A.W. Edith Chan, Andrew O’Leary, Lili Cheng, Dan Liu, Constantina Fotinou, Carla Milagre, John F. Martin, Haiyan Jia, Paul Frankel, Snezana Djordjevic, Stella E. Tsirka, Ian C Zachary and David L. Selwood. J. Med. Chem., 2018, 61, 9, 4135-4154.
5. Small Molecule Inhibitors of the Neuropilin-1 Vascular Endothelial Growth Factor A (VEGF-A) Interaction. Ashley Jarvis, Charles K. Allerston, Haiyan Jia, Birger Herzog, Acely Garza-Garcia, Natalie Winfield, Katie Ellard, Rehan Aqil, Rosemary Lynch, Chris Chapman, Basil Hartzoulakis, James Nally, Mark Stewart, Lili Cheng, Malini Menon, Michelle Tickner, Snezana Djordjevic, Paul C. Driscoll, Ian Zachary and David L. Selwood. J. Med. Chem. 2010, 53, 5, 2215-2226
Start your next project with Domainex
Contact one of our experts today