- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
kinact / KI Assay for Irreversible Covalent Compounds
Covalent inhibition is dependent on both the affinity of the compound for the binding pocket, as well as the rate of covalent bond formation by the warhead. Since this process is time dependent, the preferred measure of potency for irreversible covalent compounds is the second-order rate constant kinact/KI, rather than the IC50 value. Generation of kinact/KI data allows us to ascertain a quantitative potency metric which can be used to drive chemical optimisation during hit-to-lead and lead optimisation campaigns.
For reversible covalent inhibitors see our reversible covalent inhibitor binding assay.
Domainex’s Standard Experimental Procedure:
The target protein is incubated with the test compound at a range of concentrations at 37 °C over a period of 7 hours. The binding percentage of the compound to the protein is analysed in real time using liquid chromatography-high resolution accurate mass spectrometry (LC-HRAMS). The resulting binding percentages at each time point for each concentration are plotted to generate Kobs, then these Kobs values and the corresponding compound concentrations are plotted to calculate kinact/KI. Example data is shown in Figure 1.
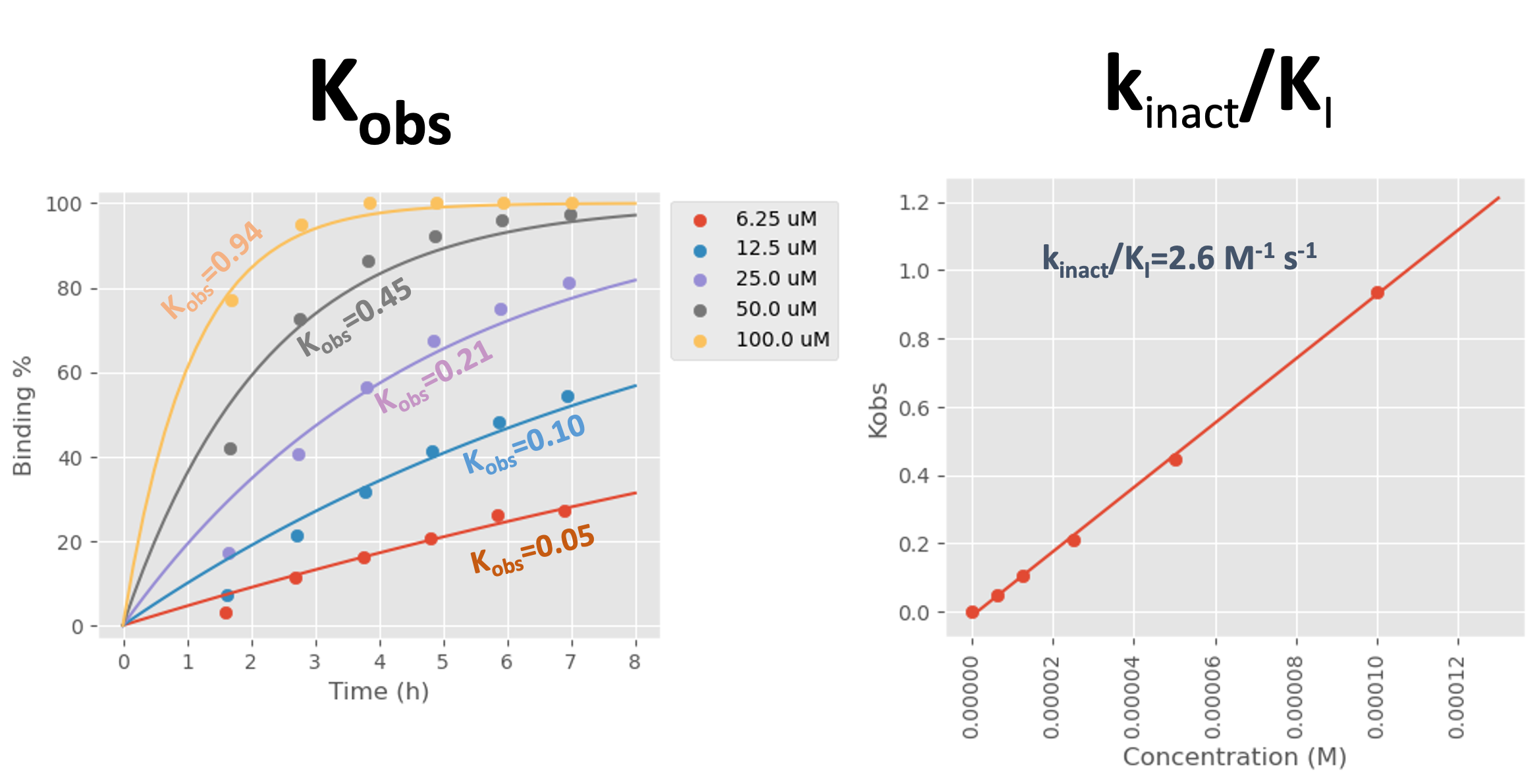
Figure 1: Kobs and kinact/KI data generated for a fragment from Domainex’s internal covalent fragment library against a client’s target protein. The binding percentage for the fragment at different concentrations was measured over a period of 7 hours
Data analysis
The increase of binding percentage over time for a compound concentration is used to calculate the Kobs value using the following equation:
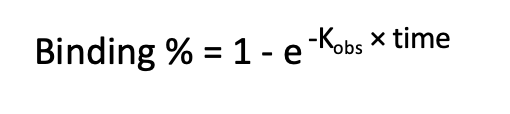
The relationship between Kobs and compound concentration is used to calculate the Kinact/KI value as illustrated in the equation below:

Deliverables
The results are reported in Excel file format including the kinact/KI value, as well as compound solubility and stability in PBS at 37 °C.
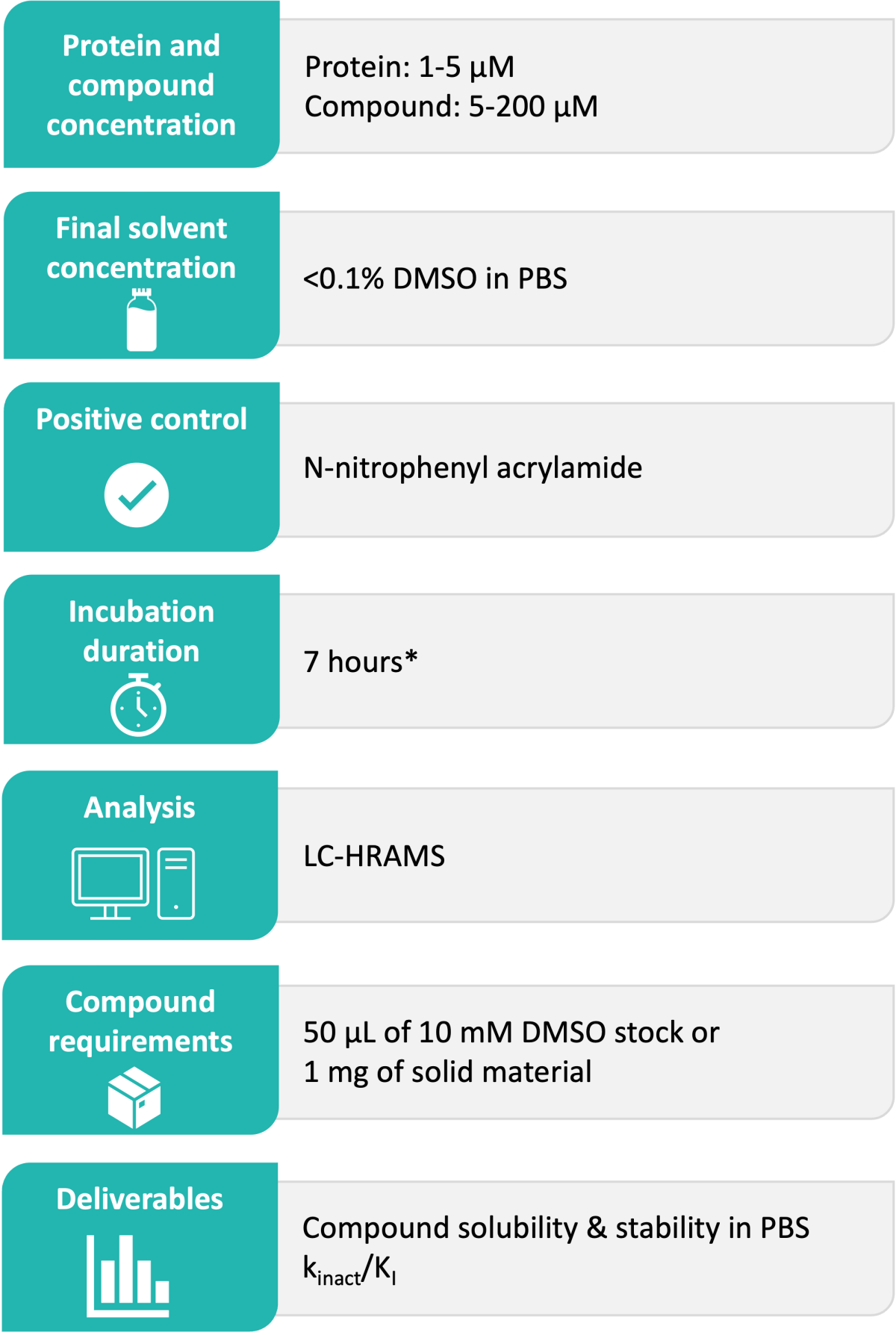
*Other options available upon request
Start your next project with Domainex
Contact one of our experts today