- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Protein Methyltransferases
PMT inhibitors as a new class of oncology drugs
Protein methyltransferases (PMTs) are a large group of enzymes that use a common mechanism to catalyse the methylation of their substrates.
PMTs bind the cofactor S-adenosyl-L-methionine (SAM) and the substrate protein to form a ternary complex. Direct transfer of the methyl group from SAM to an amino acid side chain of the substrate then occurs.1 Protein methylation of histones is used as an epigenetic2 mechanism to regulate transcription and gene stability, and recently it has also been shown to be a means by which the activity of other proteins – including enzymes such as certain kinases – may be regulated. Dysregulation of the activity of some PMTs has been shown to play an oncogenic role in a number of human cancers. Several PMT inhibitors have now entered clinical trials and Tazemetostat, an EZH2 inhibitor, gained accelerated approval from the FDA in January 2020 for the treatment of metastatic or locally advanced epithelioid sarcoma.
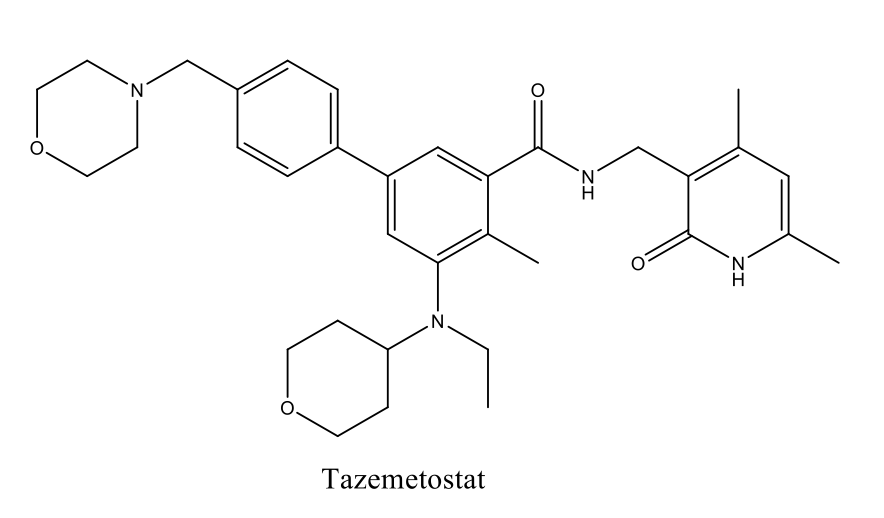
Methyltransferase Drug Discovery at Domainex
At Domainex we have worked on a number of protein methyltransferase programmes and have a particular interest in lysine methyltransferases (KMTs). Our scientists have addressed several key technical challenges associated with KMTs, including high-quality protein production, developing assays, both biochemical (see our white paper for further details) and biophysical, identifying hits (using both LeadBuilder and FragmentBuilder) and generating a number of crystal structures.
G9a
Domainex scientists have developed a lead compound targeting G9a by starting with a hit that was found by virtual screening using our LeadBuilder technology. This has low nanomolar potency and has demonstrated single-agent efficacy in difficult-to-treat cancer models. See the full case study here. We have also carried out a complementary programme of FBDD against G9a, in order to identify a back-up series (click here for further details).
If you would like to access the Domainex expertise in epigenetics to support your own programme we would be delighted to hear from you.
References
1. Human protein methyltransferases as a target class for drug discovery. Copeland RA, Solomon ME, Richon VM. Nat. Rev. Drug Discov. 2009, 8, 724–732
2. Epigenetics. J.Casadesus and M.Noyer-Weidner. Brenner's Encyclopedia of Genetics (Second Edition) 2013, Pages 500-503
Domainex press releases in the methyltransferase field
Domainex announces investment round to progress its TBK1/IKKe and epigenetic programmes
14th February 2013
Case studies
Start your next project with Domainex
Contact one of our experts today