- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Flow Cytometry
Rapid, detailed characterisation of cells and particles
Flow cytometry is used to detect and measure the physical (such as size and granularity) and chemical characteristics of particles or cell populations, for example peripheral blood mononuclear cells (PBMCs) or those in whole blood. The technique uses fluorescent reagents such as conjugated antibodies against markers of interest, DNA binding dyes or viability dyes. Alternatively, fluorescently labelled expression proteins can be characterised.
Applications of flow cytometry
There are many applications of flow cytometry including assessment of the following:
- Cell differentiation
- Cell size and granularity
- Cell population percentages
- Cell viability
- Surface receptor expression (see Figure 1 for example data)
- Intracellular protein expression
- Cell stimulation
- Cell proliferation
- Cell cycle
- Biomarkers (see Figure 2 for example data)
- Apoptosis
Advantages of flow cytometry
- Rapid analysis: Analyses subpopulations in a few minutes
- Detailed data: Able to measure multiple markers on multiple cell subsets
- Highlights non-uniformity
- Excludes debris and/or dead cells
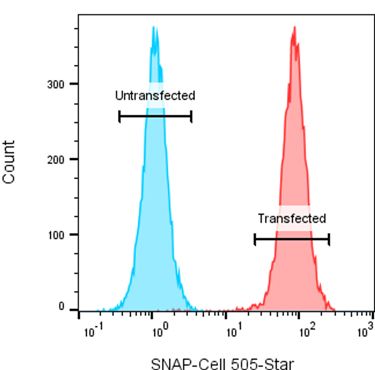
Figure 1: Example data: HEK293 cells were stably transfected with a SNAP-tag fusion protein. Transfected and untransfected cells (acting as a negative control) were stained with SNAP-Cell® 505-Star (SNAP-tag fluorescent substrate). Data shows that transfected cells (red) were SNAP-Cell 505-Star positive indicating successful transfection, whilst the untransfected control cells (blue) were negative.
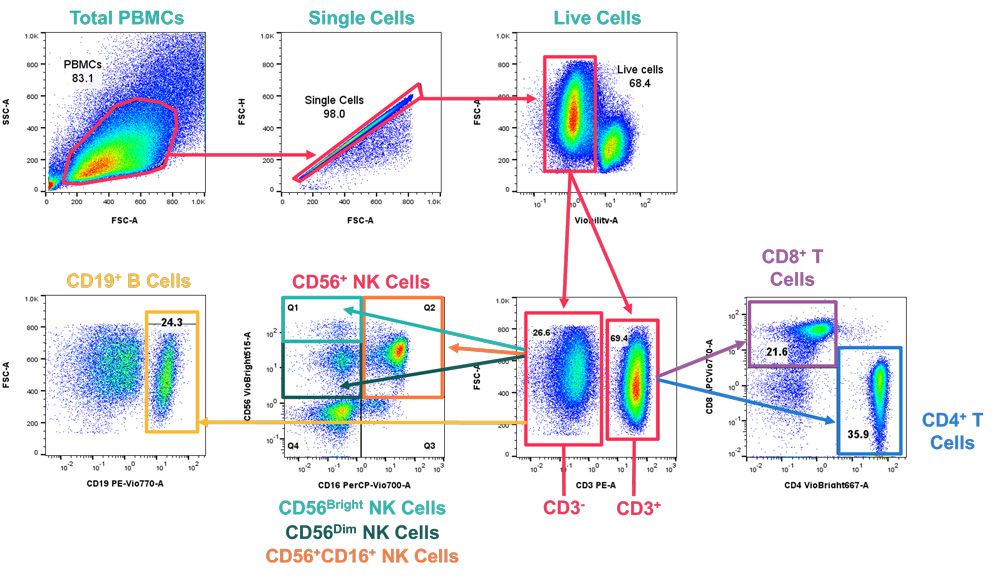
Figure 2: Example data: PBMCs were stained with an 8-parameter panel against cell lineage markers to identify different immunological subsets. Bivariate plots were used to identify cell populations; total PBMCs were gated using Forward Scatter Area (FSC-A) vs Side Scatter Area (SSC-A), with doublets then excluded using FSC-A vs Forward Scatter Height (FSC-H). Live cells were then gated into CD3- (non-T cells) and CD3+ (T cells) populations, which were further gated into a range of key immunological cell populations; CD4+ and CD8+ T cells, CD19+ B cells, and CD56+ NK Cells (further classified into 3 subpopulations; CD56Bright NK cells, CD56Dim NK cells and CD56+CD16+ NK cells).
Experienced team
Domainex has a team of highly skilled scientists with years of experience in multi-parameter panel design, sample acquisition and data analysis and interpretation. Our scientists have a range of experience with different sample types including whole blood, isolated PBMCs, enriched cell populations and cell lines, across a variety of applications such as immune cell phenotyping and binding assays. Our team are happy to discuss any flow requirements and make recommendations for the best approach for your project.
MACSQuant® X flow cytometer
We have invested in a MACSQuant® X flow cytometer from Miltenyi Biotec. The MACSQuant® X is a compact, reliable and automated flow cytometer capable of measuring up to 10 parameters (8 fluorescent channels plus Forward Scatter and Side Scatter) on cells or beads.
With an autosampler for single tubes, tube racks, 96 and 384 well plates, the MACSQuant® X is incredibly flexible with regards to sample format, whilst being built for higher throughput screening. Due to the flexibility and speed of the system, a wide range of applications are possible, ranging from single colour expression or screening studies, through to more complex multi-colour panels for cell subset identification and functional assays.
See below for further case studies.

Start your next project with Domainex
Contact one of our experts today