- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Spectral Shift and MST Services
Domainex offers world-leading services to measure interactions between ligands and biomolecules
Spectral Shift, MicroScale Thermophoresis (MST), and Temperature Related Intensity Change (TRIC) are important biophysical techniques which are widely used during all phases of pre-clinical drug discovery. As one of the earliest adopters of these techniques since 2016, Domainex boasts a substantial level of expertise in these methods, hosting the first ever Dianthus1 instrument in all of Europe.
Advantages of Spectral Shift, TRIC, and MST:
- Direct ligand binding to the target is measured in solution and therefore in its native folded state, without the risk of the binding sites being occluded by immobilisation
- Very low sample consumption
- Eliminates false positives early
- Minimal assay development time
- Compatible with a wide range of buffers
- Supports challenging biology (e.g. molecular complexes)
- Readily allows competitive binding studies to be performed
- Binding affinities (KD values) in the nanomolar to millimolar range can be determined
- Stand-alone results with the option to validate with a complementary orthogonal technique
- 384-well plate-based for compound throughput into the thousands of compounds

Spectral Shift
Spectral Shift ligand binding is monitored through changes in fluorescence. However, rather than observing changes in fluorescence intensity at a single wavelength, Spectral Shift instead monitors subtle changes in the overall emission profile of the fluorescent tag on your target of interest. Ligand binding typically results in a shift in the emission wavelength (Figure 1) through changes in the chemical environment of the fluorophore on the target, either through proximity or conformational changes (Figure 2). These subtle changes are directly monitored using an ultra-precise dual-wavelength detection system.
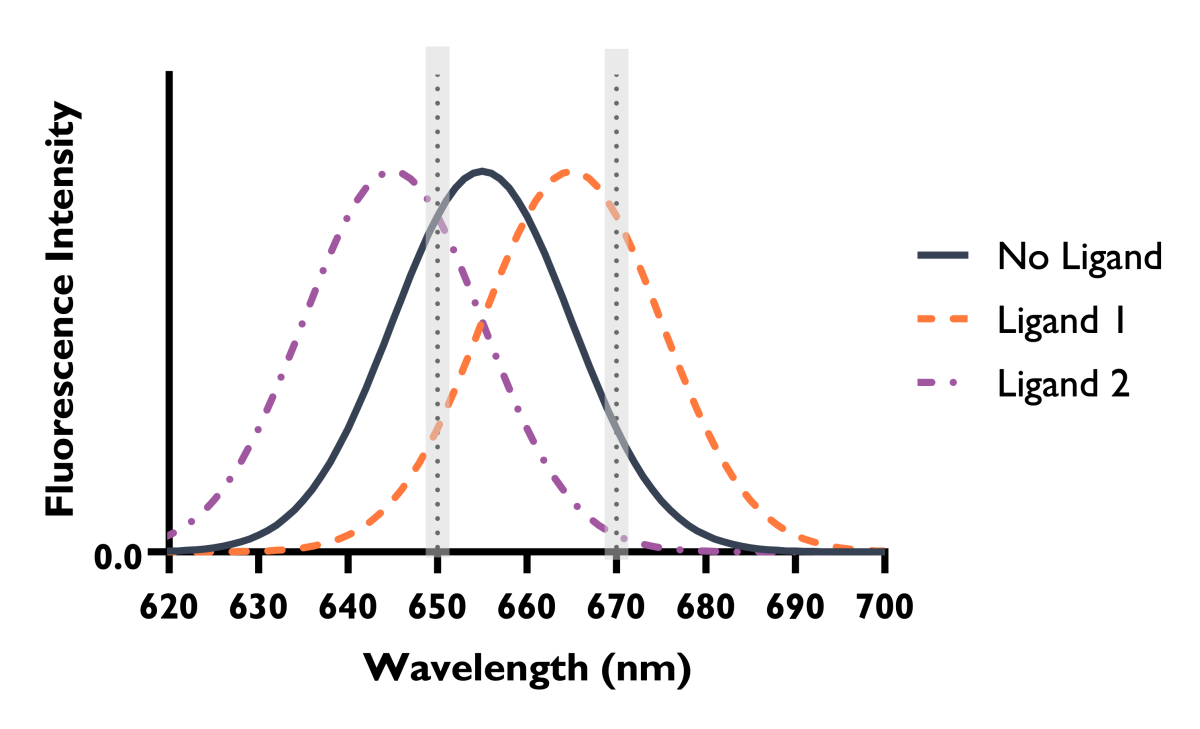
Figure 1: Shifts in the fluorophore’s emission profile, caused by ligand binding under isothermal conditions, measured by Spectral Shift.
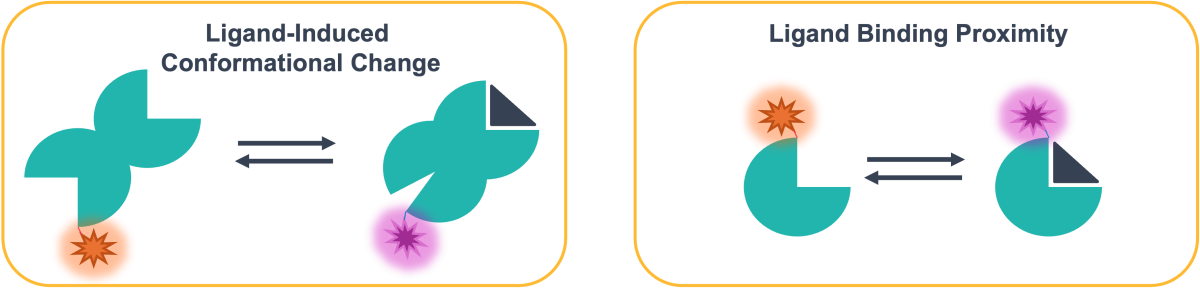
Figure 2: The impact of ligand interactions on the chemical environment of the fluorophore
Our Dianthus1 instrument includes an upgrade providing Spectral Shift capability which can record measurements individually or simultaneously alongside TRIC. As the most recent technology from NanoTemper2, Spectral Shift typically has improved signal-to-noise ratios, higher sensitivity, reduced susceptibility to the presence of insoluble aggregates, and a 15-25% faster overall run time for a 384-well plate compared to TRIC. A 384-well plate can be screened in less than 35 minutes when taking Spectral Shift measurements alone or less than 80 minutes for Spectral Shift and TRIC simultaneously. We can screen several thousand samples in 24 hours, meaning the technique is suitable for use in medium-throughput screening.
Domainex has applied Spectral Shift technology to fragment screening, RNA binding, probe displacement, bifunctional ligands (e.g. ternary complexes with protein degrader compounds), molecular glues and membrane proteins.
MST and TRIC
MST is capable of measuring the strength (affinity, KD) of interactions by measuring the motion of a fluorescently-labeled molecule along a temperature gradient. When a ligand (e.g. small molecule, antibody, DNA aptamer, etc.) binds to a fluorescently labeled target, changes in the charge, size, or hydrodynamic radius occur, which ultimately effects the rate of diffusion along the thermal gradient (thermophoresis). A standard experiment typically includes a fluorescently labeled target held at a constant concentration and a titration of ligand with all other conditions remaining the same. Thus, changes in thermophoresis reflect changes in physicochemical properties as the result of the binding interaction. Changes measured during titration of the ligand are recorded and directly correlate to the relative bound:unbound state of the target. Domainex has invested in a Monolith NT.Automated high-end instrument from NanoTemper2, a 96-capillary-based system, for this exact purpose.
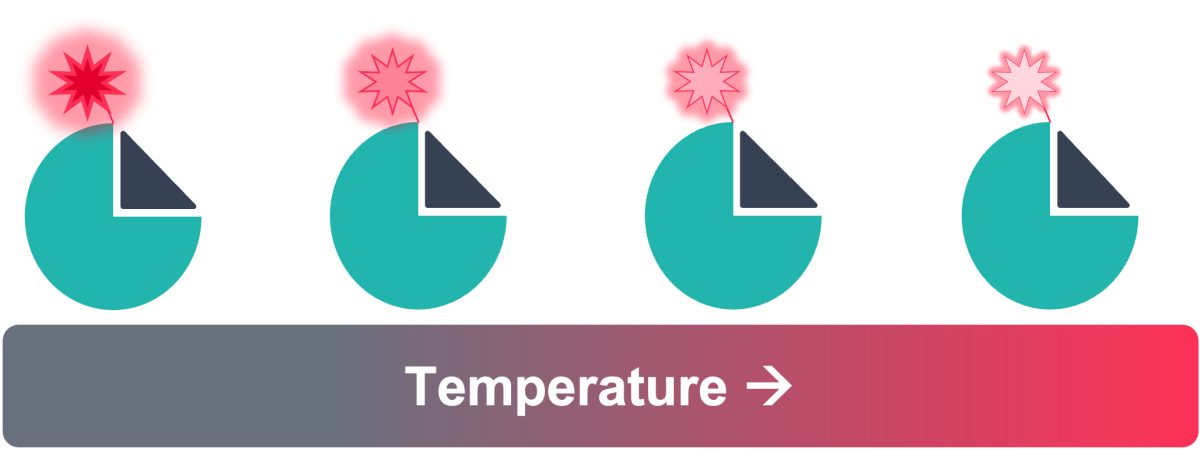
Figure 3: Principle of TRIC – fluorescence intensity changing with increasing temperature, which is affected by ligand binding
TRIC is an additional component of thermophoresis, whereby the fluorescence intensity of a fluorophore changes depending on local temperatures in solution - typically there is a decrease in fluorescence with increasing temperature (Figure 3). However, in addition to changes in fluorescence due to the physical environment, TRIC is strongly influenced by the chemical environment, which can be altered upon the binding of a ligand to the target near the fluorescent label, or by a conformational change of the target as a result of binding to the target elsewhere (Figure 2).
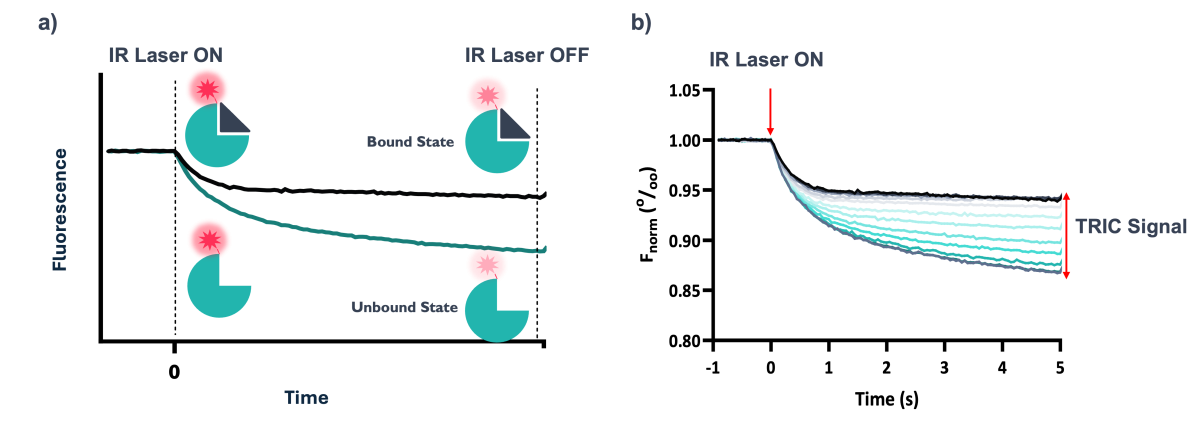
Figure 4: a) Representation of the effect of ligand binding on the TRIC signal. In this example, when ligand is bound the reduction in fluorescence is less significant over time compared to the unbound state. b) Normalised fluorescence over the course of a standard TRIC experiment
Labelling Strategies
A wide range of labelling strategies, both covalent and affinity based, are readily available to investigate your system of interest.
Covalent based dyes:
- Maleimide
- NHS
- SNAP-Tag
Contact one of our experts today to find out more information about our services.
Affinity based dyes:
- Biotin
- His-Tag
- Human FC
Start your next project with Domainex
Contact one of our experts today