- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- DMPK Services
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Neuroinflammation
Development of assay systems to aid in the fight against chronic neuroinflammation
Inflammatory responses are critical for survival; however, aberrant inflammation is implicated in a variety of neurodegenerative, cardiovascular, autoimmune, and metabolic diseases.
The central nervous system (CNS) has a separate immune state to the rest of the body, initiating its own neuro-inflammatory response via microglia. Due to the CNS’s poor repair mechanisms, immune responses must be terminated to maintain homeostasis. Persistent insult, or inadequate termination, leads to chronic neuroinflammation, a continuous and self-exacerbating cycle.
Chronic neuroinflammation has been identified as a driving force in many brain pathologies including dementias, traumatic brain injury and stroke. Drugs that inhibit abnormal inflammatory responses (e.g. nucleotide-binding oligomerization domain-like receptors family pyrin domain containing 3 (NLRP3) inflammasome inhibitors) have thus become highly sought after, due to their potential to modulate multiple disease-states.
Domainex has a team of highly skilled scientists with world-class knowledge and experience in drug discovery for inflammatory conditions, including neurodegenerative and neuroinflammatory disorders. Our scientists have a wide range of experience and expertise and employ in house state-of-the-art technologies to solve complex research challenges. We have developed a comprehensive suite of assays to enable the profiling and development of novel compounds targeting inflammatory and neuroinflammatory targets.
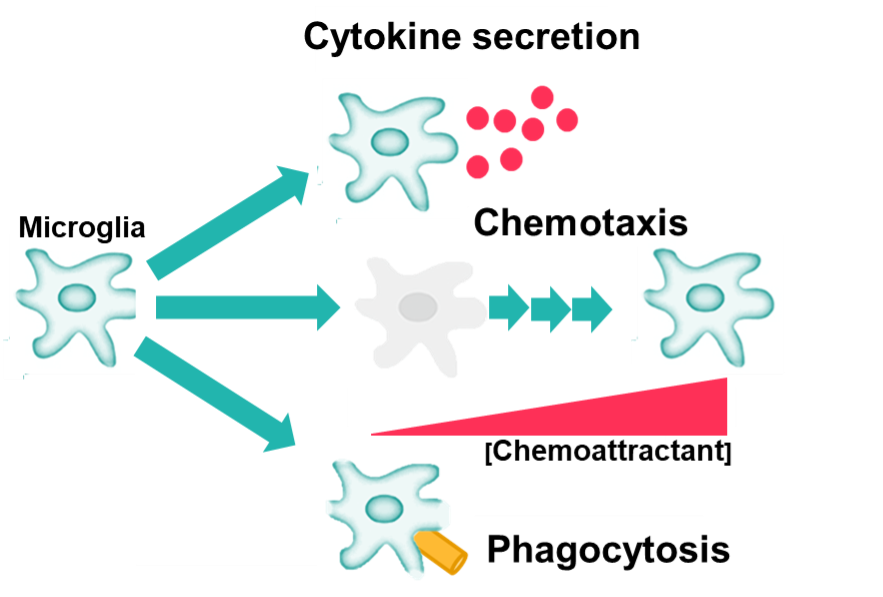
Figure 1: Schematic diagram showing steps involved in neuroinflammation-mediated neurodegeneration. Microglia, the resident innate immune cells of the brain, are activated by a number of factors such as normal aging, dementia, stroke, infections, pathological stimuli and more. Activated microglia can phagocytose foreign organisms and engulf unwanted neuronal debris, produce cytokines (proinflammatory and neurotoxic mediators), and also migrate (chemotaxis) towards a lesion/inflammation site. Chronic activation of microglia may cause inflammation-mediated neurotoxicity and progressive neurodegenerative insults to brain and CNS.
Chemotaxis
Macrophages and microglia both possess an inherent ability to migrate towards a chemoattractant (e.g. at the site of injury). Domainex has developed assays allowing the measurement of the induction and inhibition of chemotaxis in both THP-1 cells (differentiated into M0 macrophages) and induced pluripotent stem cell (iPSC)-derived microglia, using an Incucyte® Live-Cell Analysis System.
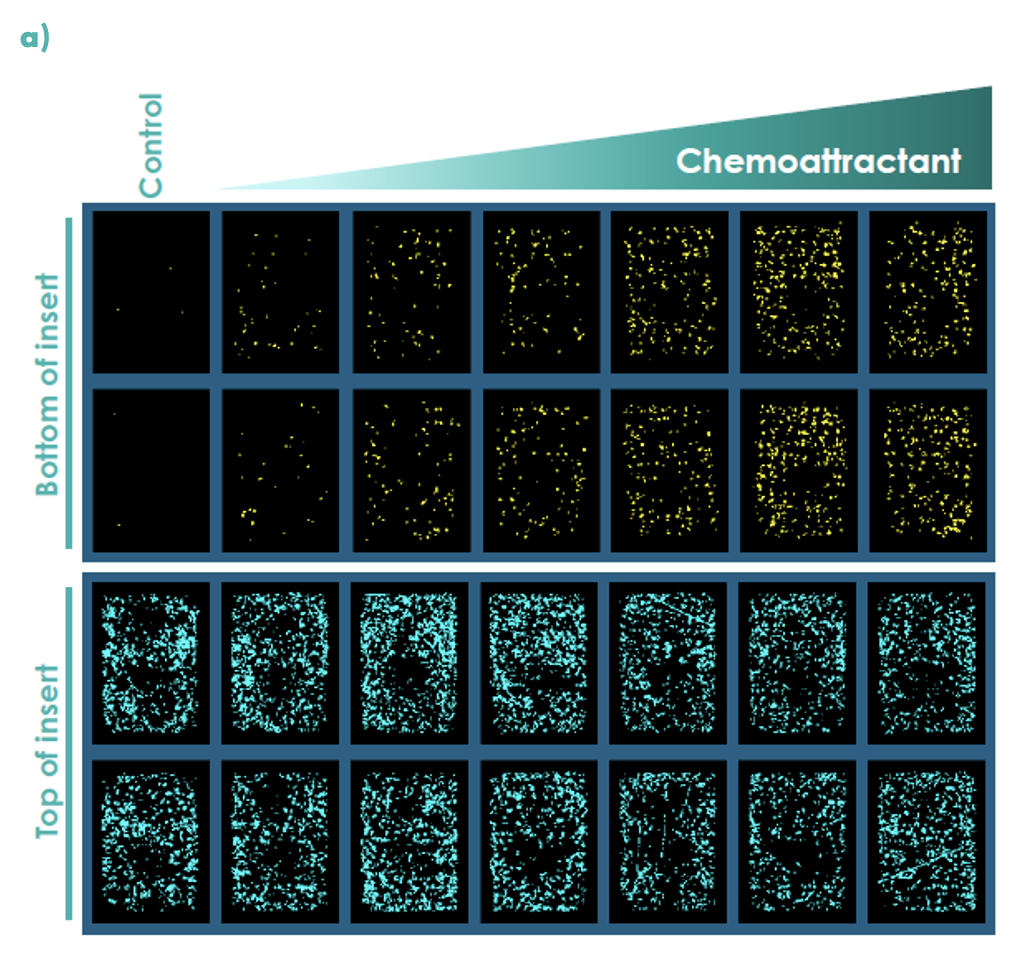
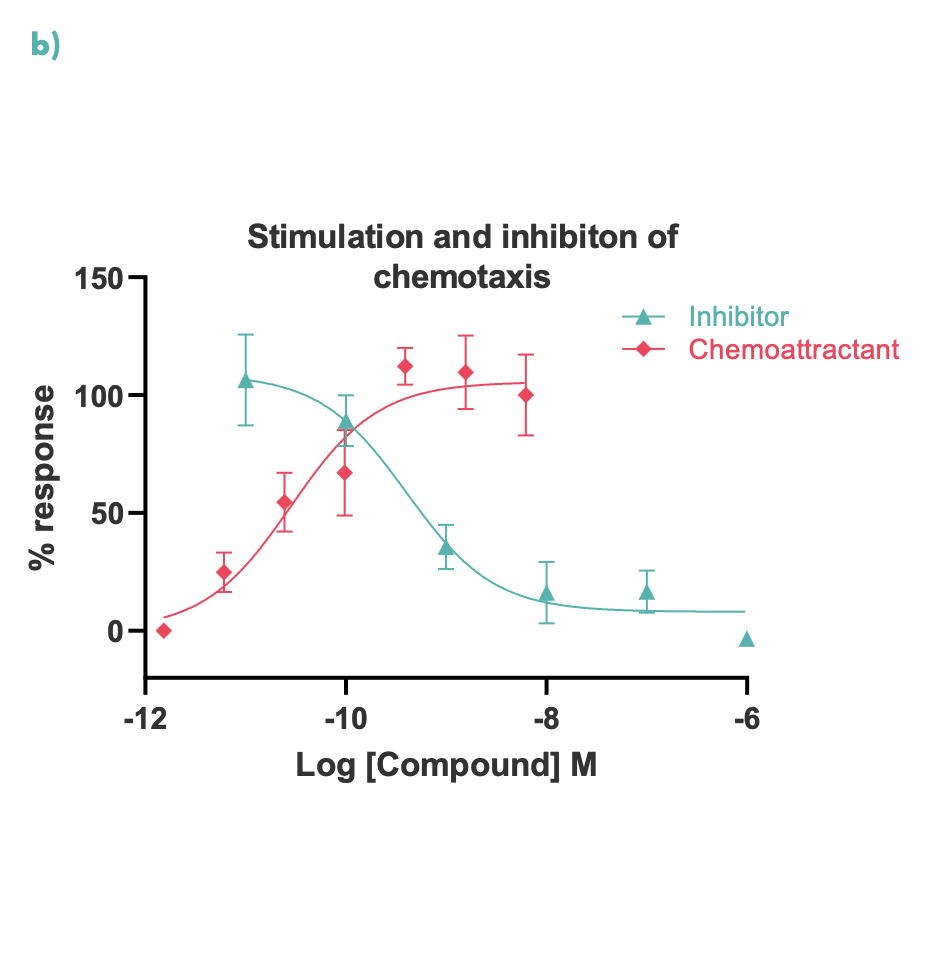
Figure 2: Chemotaxis was measured in real-time using an Incucyte S3 for M0 differentiated THP-1 cells; (a) representative Incucyte images, (b) compound concentration-response curves for cell migration were measured at 48 hours.
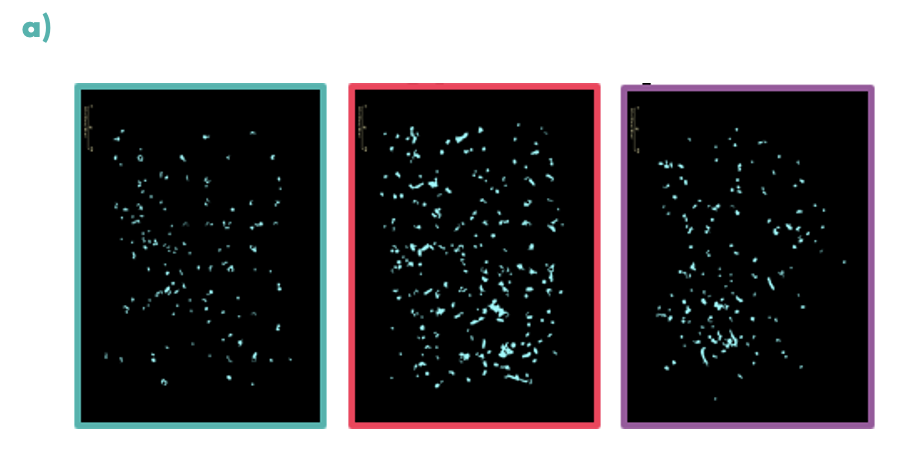
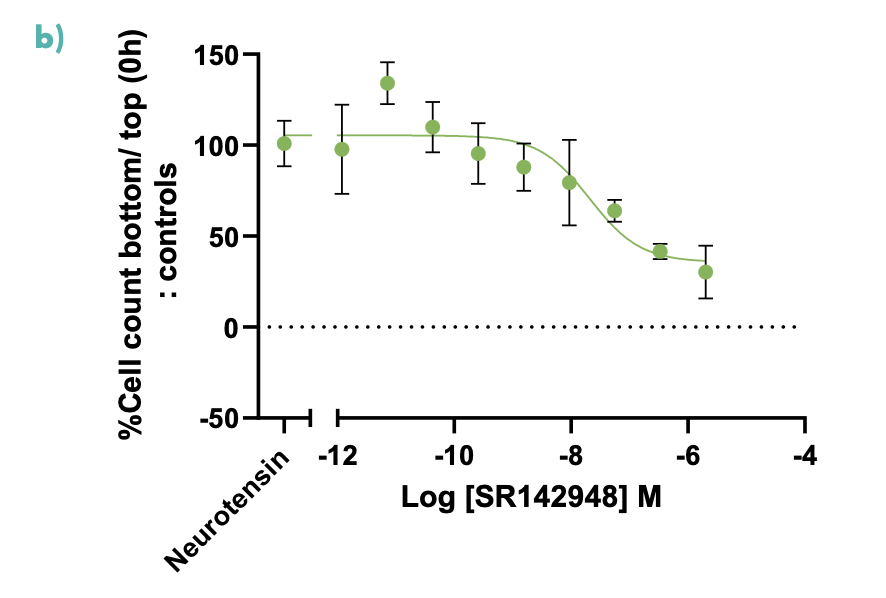
Figure 3: Chemotaxis was measured in real-time using the Incucyte S3 for microglia; (a) coloured boxes show representative images of the analysis mask at low (teal), medium (pink) and high (purple) concentrations of neurotensin, demonstrating a drop-off in response at high concentrations, (b) the graph shows iPSC microglia treated with a concentration response curve of the neurotensin receptor inhibitor SR142948. The inhibitor successfully decreased the neurotensin induced chemotaxis of iPSC microglia in a concentration-dependent manner.
Inflammasome
NLRP3 is a key regulator of inflammation. Hallmarks of NLRP3 inflammasome activation include the release of proinflammatory cytokines such as interleukin-1β (IL-1β), and cell death (pyroptosis). Domainex has developed robust screening assays measuring inflammasome activation in THP-1 cells (differentiated into M0 macrophages), whole blood, and iPSC-derived microglia.
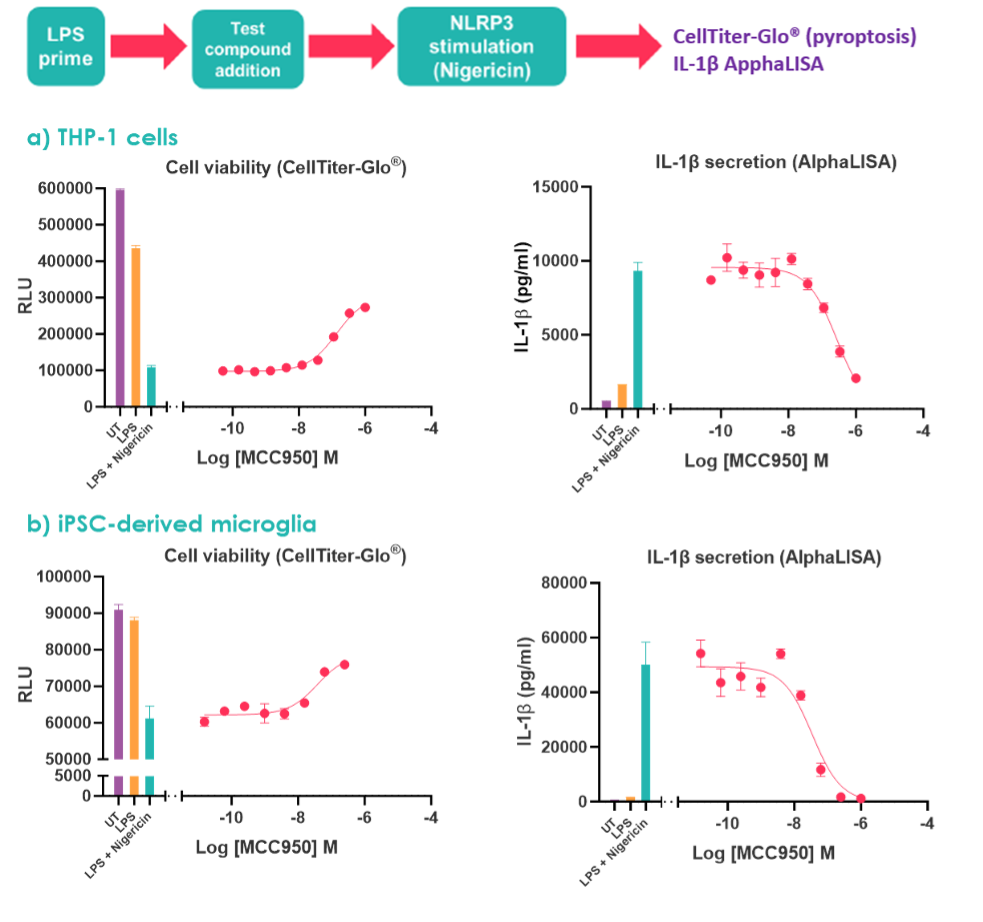
Figure 4: To stimulate the NLRP3 inflammasome (a) M0 differentiated THP-1 cells and (b) iPSC microglia were treated with LPS +/- nigericin. Concentration-response curves were performed with the NLRP3 inhibitor MCC950. UT denotes untreated.
Phagocytosis
A key process in an inflammatory response is the clearing of pathogens, cell debris and apoptotic cells via phagocytosis. Domainex has developed assays to quantify THP-1-mediated phagocytosis of apoptotic Neuro-2a cells labelled with pHrodo™ Red dye. pHrodo™ fluorescence increases once inside the acidic environment of the phagosome and thus acts as a marker of phagocytosis.
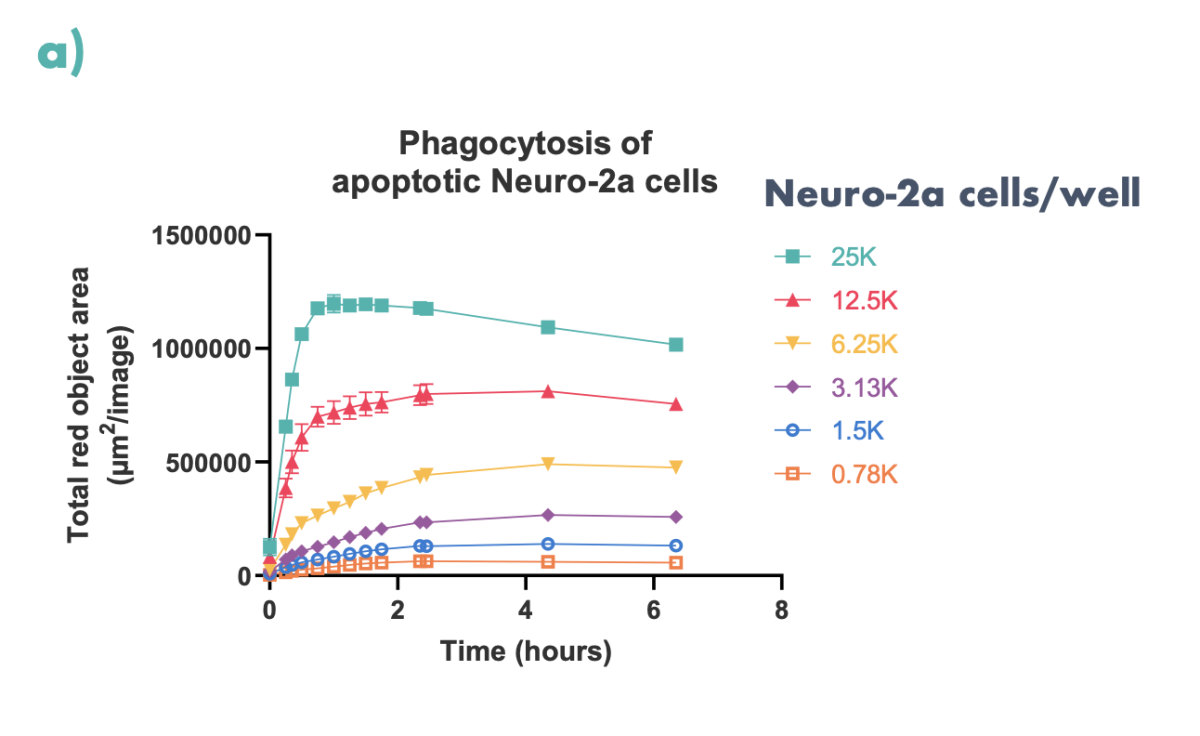
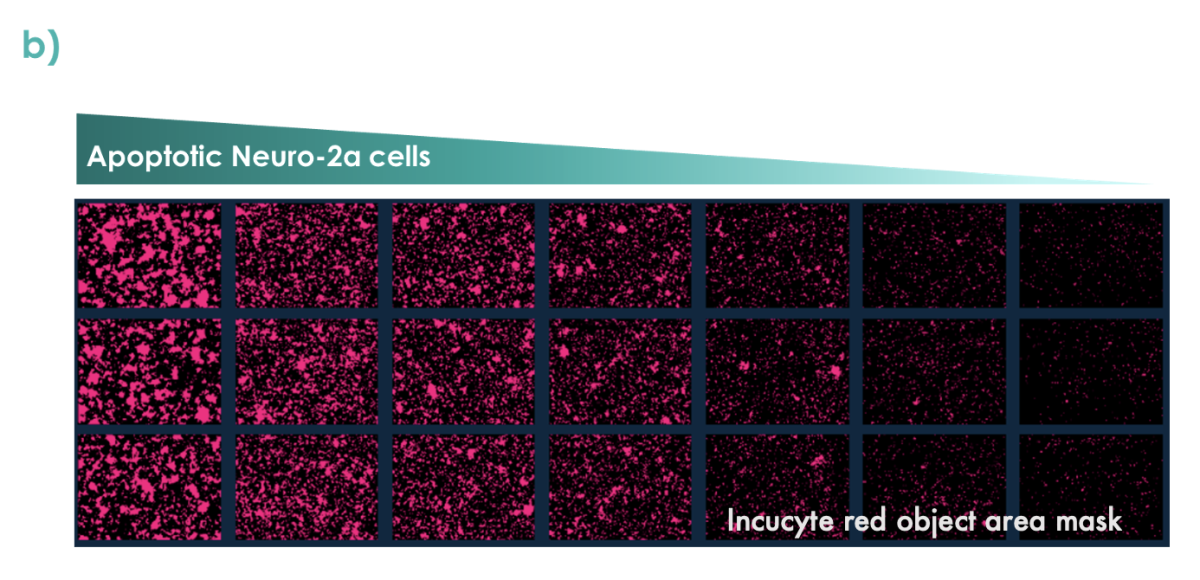
Figure 5: Apoptotic Neuro2a-cells (pre-treated with staurosporine and labelled with pHrodo™ Red) were co-cultured with M0 differentiated THP-1 cells. (a) Phagocytosis was quantified over time using the Incucyte S3. (b) Representative images of phagocytosed Neuro-2a cells at 6.5 hours are shown.
Microglia, much like macrophages, phagocytose cell debris, plaques, and infectious agents to destroy them. pHrodo™ Green S. aureus BioParticles™ were employed to study the impact of neurotensin on this action. pHrodo™ fluorescence increases once inside the acidic environment of the phagosome and thus acts as a marker of phagocytosis. When neurotensin and BioParticles were added simultaneously little difference in phagocytosis was observed (data not shown), however, literature suggested TNFα is required to increase microglia phagocytosis [1]. Cells were therefore pre-treated with neurotensin before BioParticle addition to allow for TNFα release.
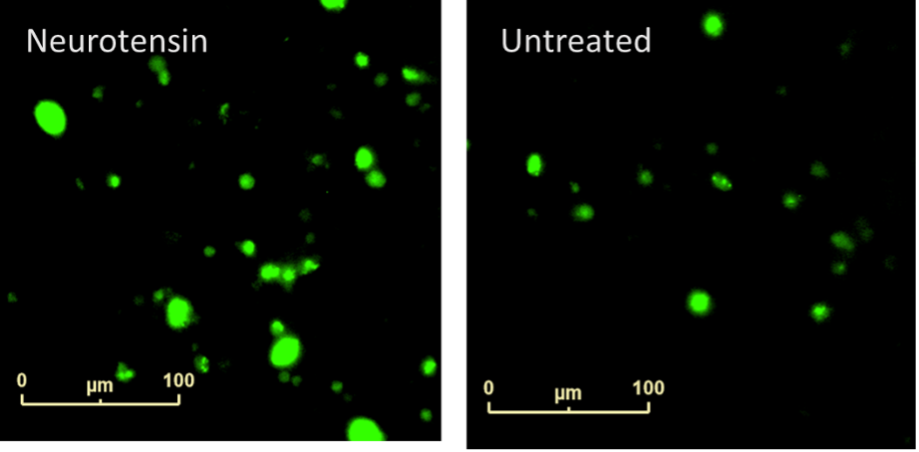
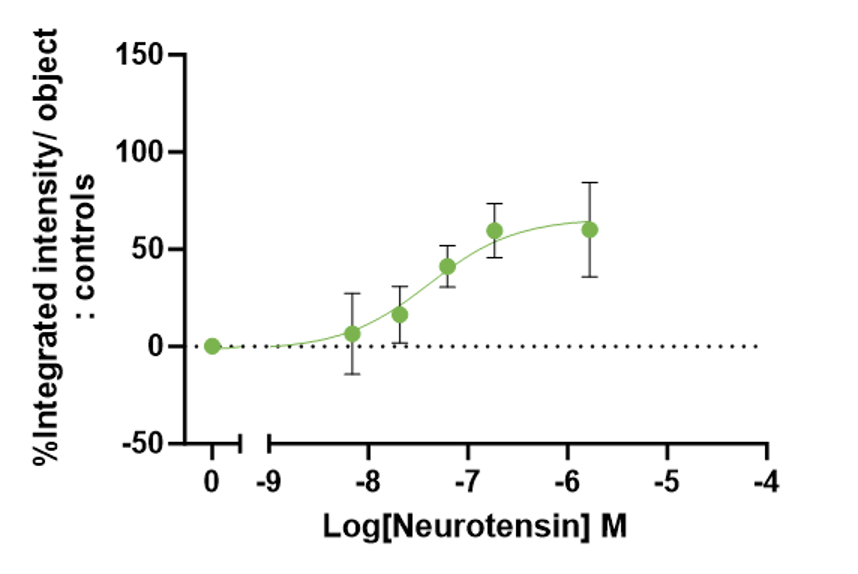
Figure 6: Neurotensin-induced phagocytosis of iPSC-derived Microglia. A clear concentration dependent increase in neurotensin-induced phagocytosis was seen at 21 hours.
Cytokine release
Microglia will release cytokines at the site of injury to attract and activate other microglia. AlphaLISA technology was employed to measure release of TNFα and IL-1β cytokines from iPSC microglia in response to neurotensin. iPSC microglia remain in culture after supernatant sampling allowing for multiplexing with other readouts.
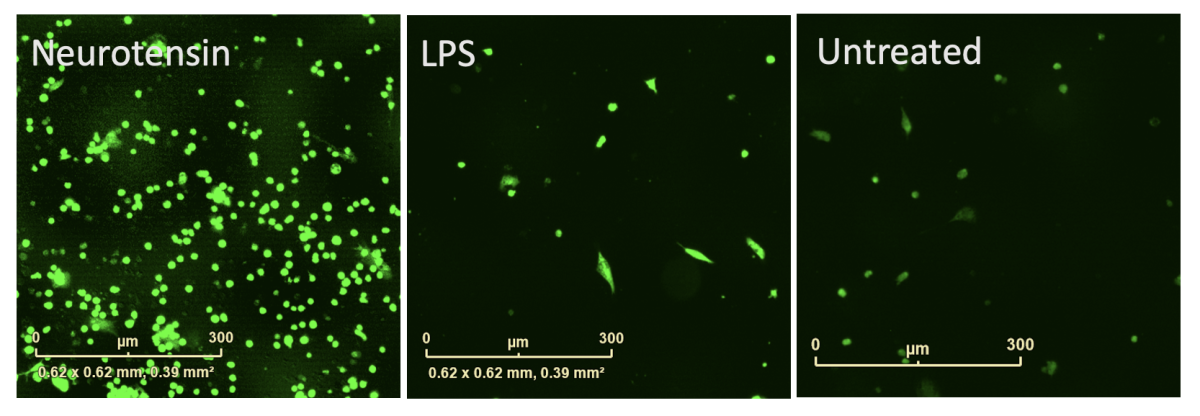
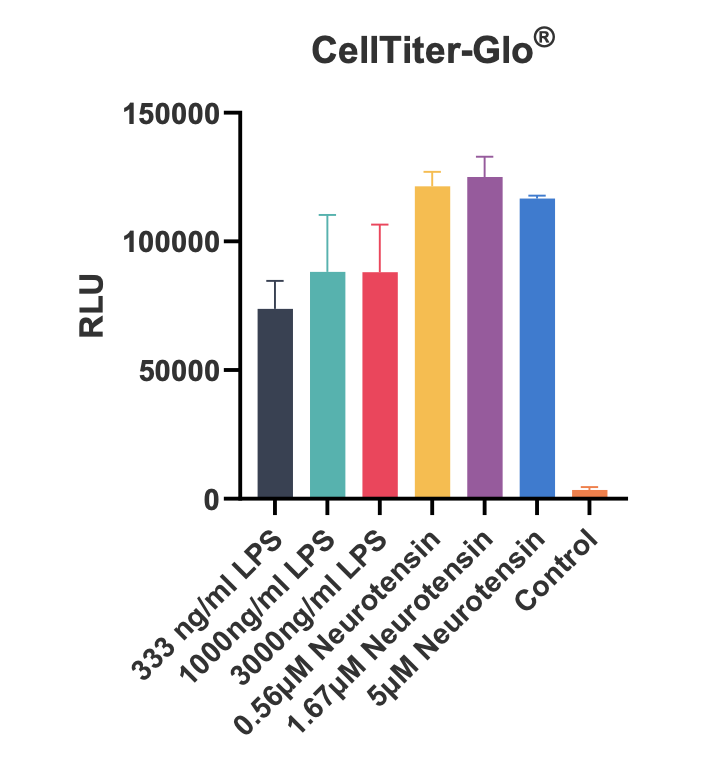
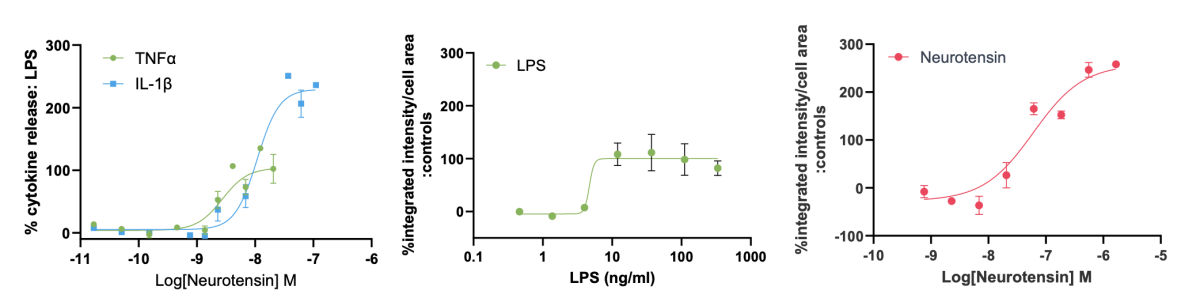
Figure 7: Reactive oxygen species (ROS) were measured using the live cell dye dichlorodihydrofluorescein diacetate (DCFDA) and cell viability by CellTiter-Glo®. iPSC microglia produced TNFα and IL-1β cytokines and increased ROS production in response to neurotensin without impacting cell viability. IL-1β and ROS production was greater than with LPS.
In summary, these robust assays can be performed routinely, allowing progression of hit-to-lead and lead optimisation programmes, ultimately assisting in the nomination of preclinical candidates. Furthermore, the assays can be performed in a variety of relevant cellular models, and further tailored, as required, to the needs of your project.
Reference
[1] Neniskyte U, Vilalta A, Brown GC. Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. FEBS Lett. 2014 Aug 25;588(17):2952-6. doi: 10.1016/j.febslet.2014.05.046. Epub 2014 Jun 6. PMID: 24911209; PMCID: PMC4158418.
Start your next project with Domainex
Contact one of our experts today