- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- DMPK
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Plasma Protein Binding Assay
Pharmacokinetic and pharmacodynamic properties of test compounds are affected by the unbound (free) plasma concentration, as compound bound to plasma proteins is unable to reach an effective concentration at the site of action. It is highly important to understand the degree of plasma protein binding (PPB) as this not only impacts the efficacy of your drug candidate, but also influences potential toxicity. Domainex assesses test compound plasma protein binding by equilibrium dialysis using Rapid Equilibrium Dialysis (RED) devices.
Domainex’s Standard Experimental Procedure:
Plasma protein binding is measured using a RED method and RED device inserts. Test compounds and assay standards are spiked into plasma and dialysed against phosphate buffered saline (PBS) at 37 °C for four hours. Following incubation, samples from each chamber are matrix matched and compounds extracted using organic solvent. Samples are diluted further for Ultra-High Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS) analysis. Example data is shown in Figure 1.
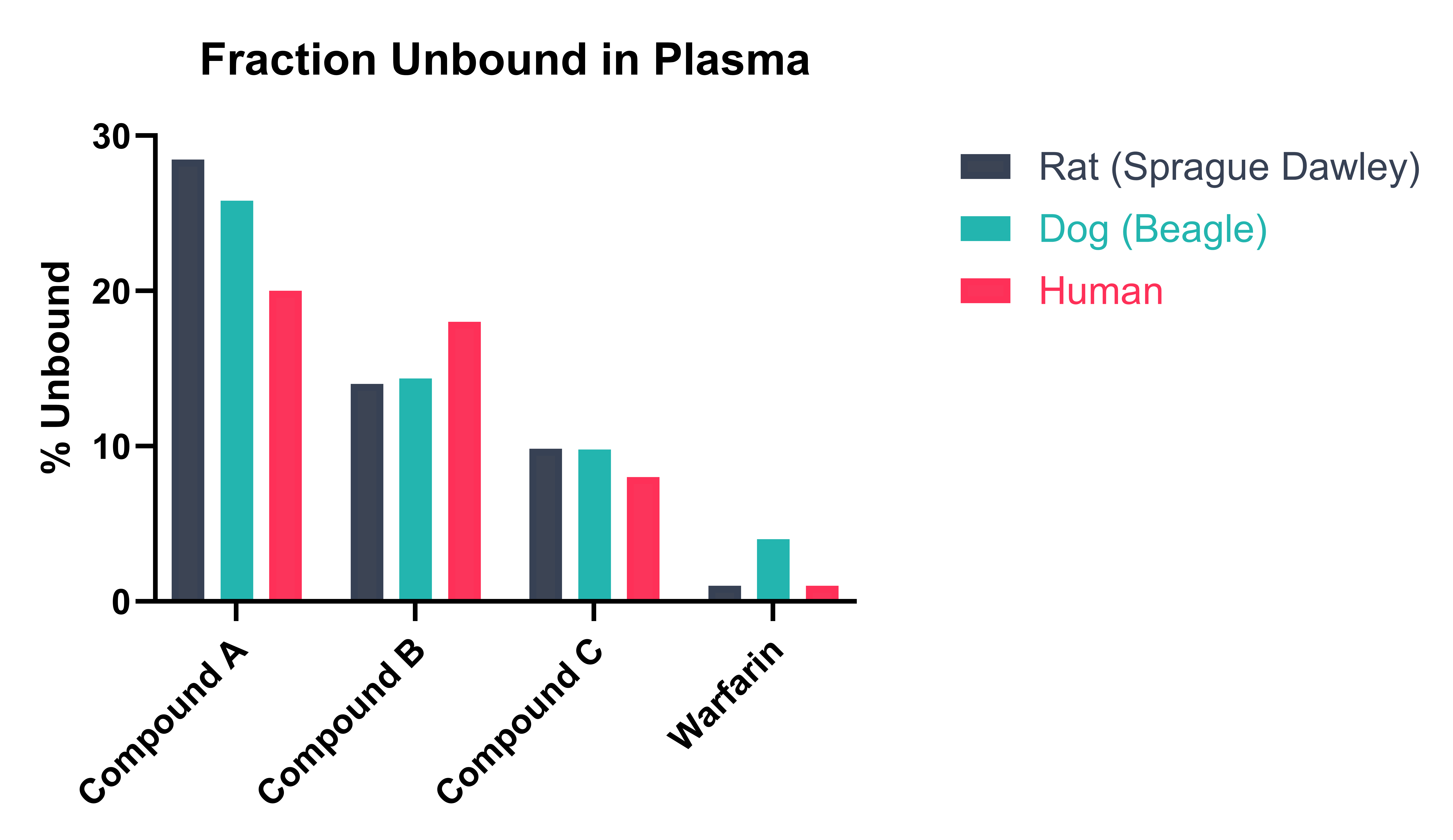
Figure 1. Example fraction unbound results for three test compounds and a control (warfarin) in different species.
*Other options available upon request
Data Analysis:
Extracts are analysed using Waters Xevo TQ-S micro, Xevo TQ-S or Xevo TQ-XS mass spectrometers, paired with a Waters ACQUITY UPLC H-Class system. Triple quadrupole mass spectrometers operated in multiple reaction monitoring (MRM) mode, provide measurements with excellent sensitivity, selectivity and reproducibility.
Deliverables:
The percentage of unbound compound (compound free) is calculated using the following equation:

The results are reported in Excel file format as compound bound (%), compound free (%) and mass balance (%). Any relevant comments about compound stability, solubility or binding are also included in the report.
Typical turnaround time from receipt of test compounds to release of data is ten working days or less.
Start your next project with Domainex
Contact one of our experts today