By Gabriel Negoita-Giras (Team Leader, Medicinal Chemistry)
Werner helicase (WRN) is an enzyme that helps to maintain the structural integrity of DNA. It achieves this by unwinding and separating double stranded DNA as part of the cellular machinery maintaining genome stability. Therefore, WRN is recognised as a precision oncology target, having a synthetic lethality relationship with microsatellite-high instability (MSI-H) tumours.
A successful approach to WRN inhibition relies on covalent engagement of Cys727 of WRN, with advanced inhibitors (such as VVD-133214) achieving this using a vinyl sulphone electrophile (an unprecedented group in marketed drugs). In this blog, we highlight recent work by GSK1 to discover novel WRN Cys727 binders bearing a better characterised acrylamide group.
To this end, they developed an intact protein LC-MS screening platform to identify covalent binding to WRN. Using this platform, they identified hits 1 and 5 which showed single labelling of WRN (Figure 1) while also being within an accepted safety range for reactivity (GSH t1/2).
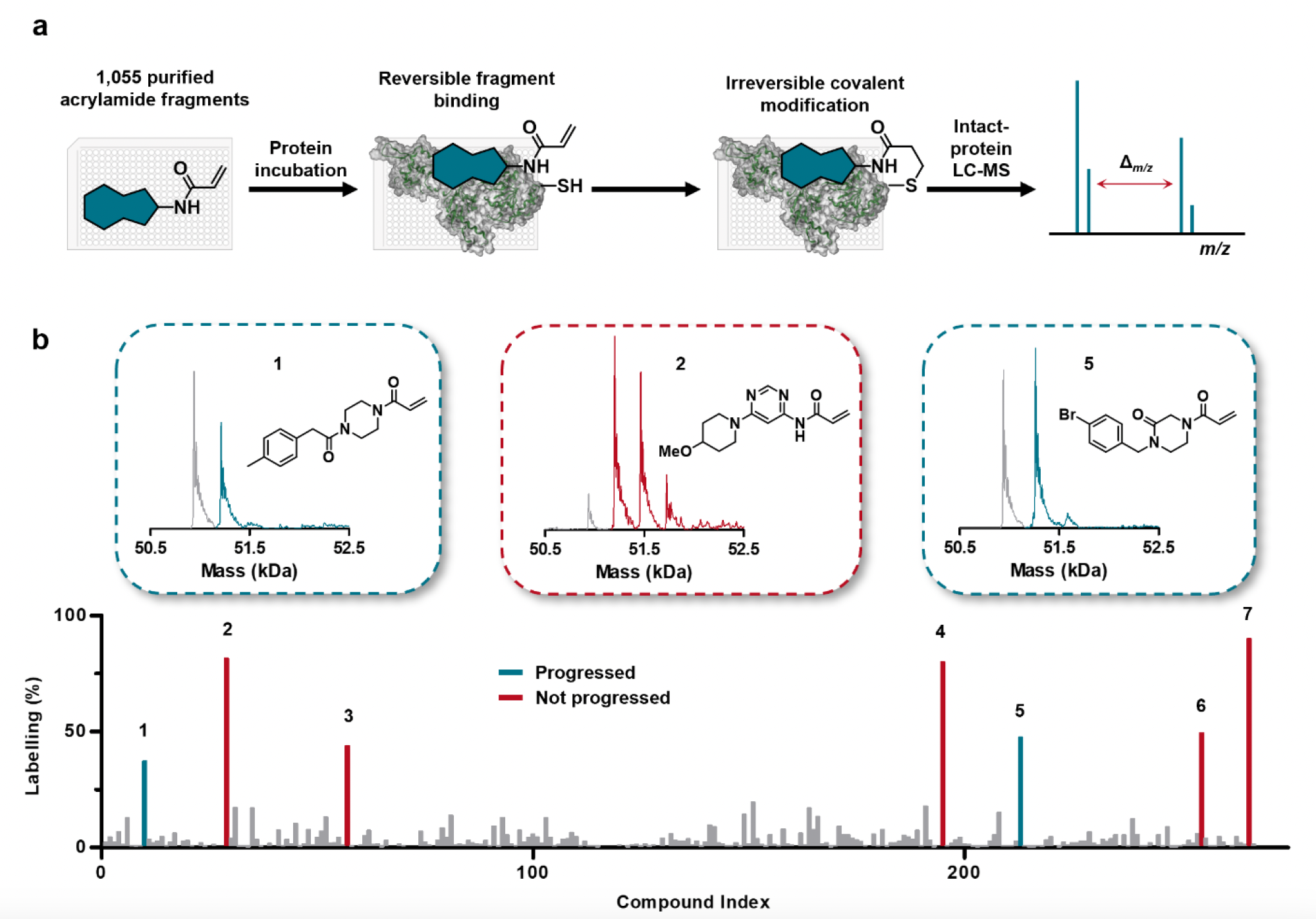
Figure 1. a. Schematic highlighting GSK’s intact protein LC-MS platform; b. Extent of labelling of WRN and mass spectrometry data for identified hits. Images are taken from Bush et al.1 with permission from the authors.
These hits were shown to covalently bind Cys727, however, only 1 showed selectivity for WRN within the RecQ helicase family, while 5 was more promiscuous.
In parallel, a FRET assay was established to assess WRN helicase activity in the presence of compound. This revealed a pIC50 of 4.8 for 1 after preincubation with the enzyme for 4 hours. The kinact/Ki was also determined to be 3.7 M-1s-1.
To further enhance the potency as well as the kinact/KI for 1, the team adapted their LC-MS screening platform for direct-to-biology (D2B) use and deployed it in a modular fashion to explore chemical space around the compound. This allowed them to quickly establish and expand the structure activity relationships (SAR) and led to the discovery of (R)-11 with a WRN pIC50 of 6.0 and a kinact/KI value of 21 M-1s-1 (Figure 2).
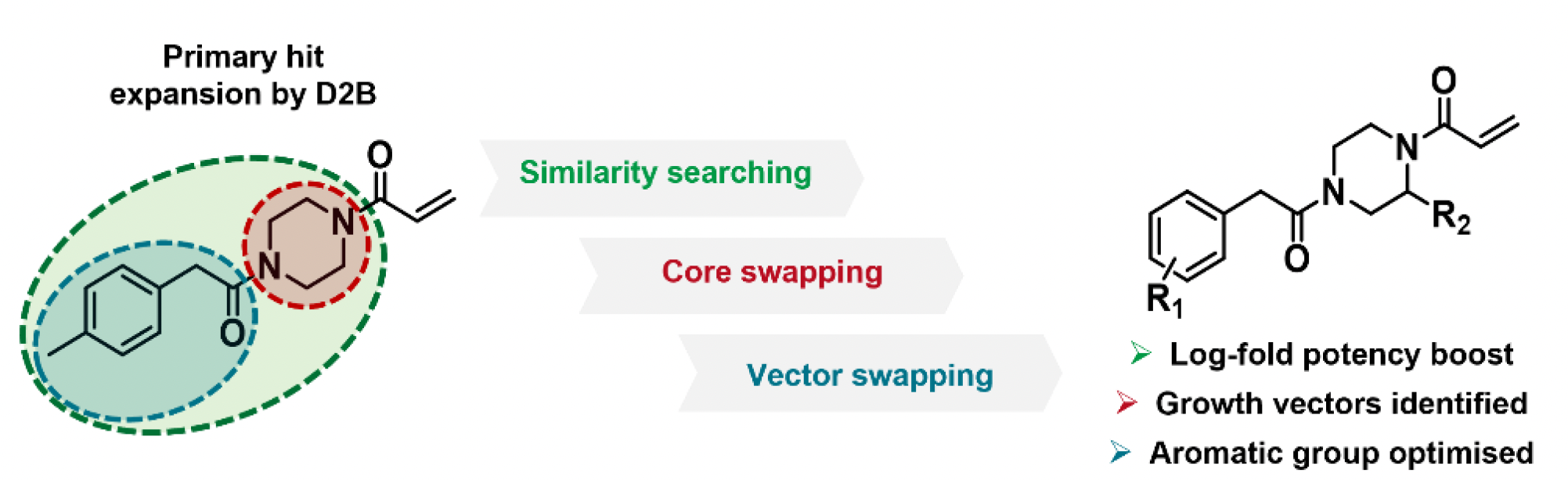
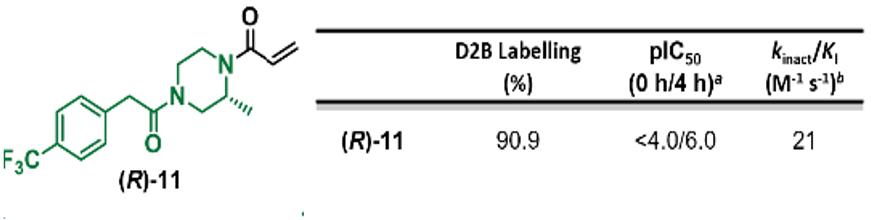
Figure 2. Schematic of areas of SAR expansion and structure and assay data for (R)-11. Images are taken from Bush et al.1 with permission from the authors.
The crystal structure of (R)-11 with WRN was solved and this confirmed the covalent attachment to Cys727 while also identifying a protein conformation that was not observed for clinical agent VVD-133214. Furthermore, it revealed a growth opportunity (off the methyl on the piperazine ring).
To explore this, a further D2B campaign was launched that identified (S)-27 as a superior analogue with a WRN pIC50 of 7.5 and a kinact/KI value of 1300 M-1s-1 (Figure 3).
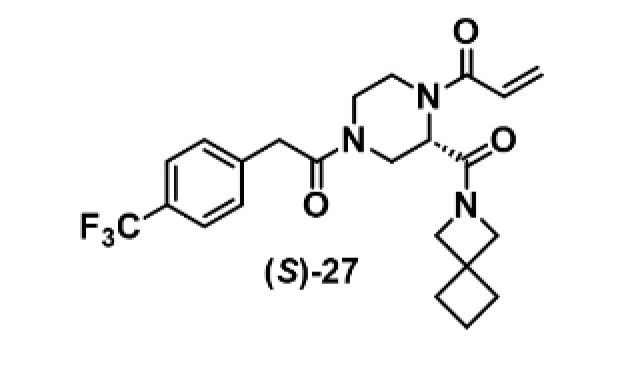
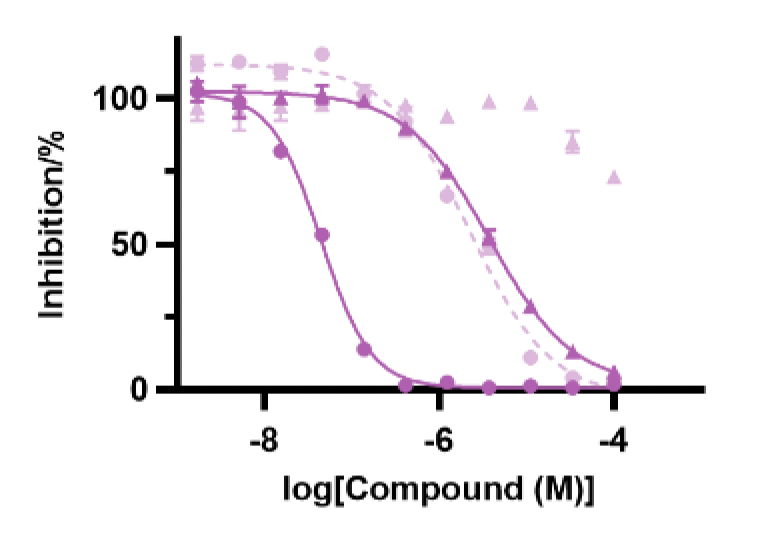

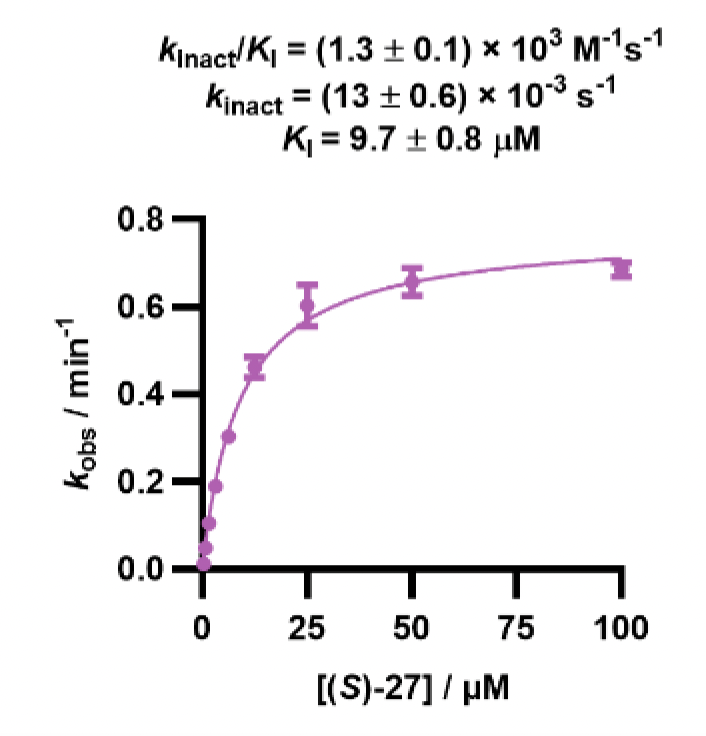
Figure 3. Structure of (S)-27 and the associated pIC50 and kinact/KI assay data. Images are taken from Bush et al.1 with permission from the authors.
Chemoproteomic profiling revealed that (S)-27 engaged only two cysteines in the proteome with >50% occupancy, one of them being WRN Cys727. Cell viability was assessed in MSI-H and microsatellite stable (MSS) cell lines (SW48 and SW620) and while a dose-response curve was observed for the former (pIC50=5.0), the compound was inactive in the MSS cell line.
In summary, by employing a D2B screening platform modularly, the GSK team achieved a rapid SAR expansion in a resource and time efficient manner. They established multiple single step and multi-step routes that were high throughput and D2B compatible, thus synthesising >1,200 compounds, while only purifying approximately 20 analogues. This yielded (S)-27, an acrylamide covalent inhibitor, which achieved a 400-fold improvement in IC50 and kinact/KI over hit 1, while also showing activity in cellular assays. Undoubtably, (S)-27 will serve as a valuable tool compound to explore the role of WRN in various cancers.
At Domainex, we are also passionate about high-throughput experimentation (HTE) and direct to biology (D2B) and are experts at identifying and progressing hits quickly to potent and effective therapeutic agents. If you would like to get in touch and discuss how Domainex could help with your project, please contact us here.
Reference
- Jacob T. Bush, et al. ‘Direct-to-biology’ drives optimisation of a cell-active covalent inhibitor of WRN helicase. ChemRxiv. 2025; doi:10.26434/chemrxiv-2025-tvdzn This content is a preprint and has not been peer-reviewed