By the Domainex Synthesis Group (Alicia Galván Álvarez, Claire Sear, Hugh Tawell, Tenin Traore, Andrew Jones, Jonas Calleja and David Gibson)
In the latest edition of our blog series, we have focused on the following two recent publications, one describing the oxidative cleavage of alkenes (analogous to ozonolysis) using mild conditions and the other performing hydrogen atom transfer from unactivated alkanes using a cheap and readily available photocatalyst.
- Photoexcited nitroarenes for the oxidative cleavage of alkenes. Danieli Leonori et al, Nature, 610, 81-86, 2022.
- Brønsted acid-enhanced direct hydrogen atom transfer photocatalysis for selective functionalization of unactivated C(sp3)–H bonds. Jie Wu et al, Nat. Synth (2022).
Photoexcited nitroarenes for the Oxidative Cleavage of Alkenes
The oxidative cleavage of carbon-carbon double bonds is an important reaction at the discovery, process or bulk chemistry scale. Whether for converting feedstock alkenes into more useful materials or for medicinal chemistry programmes, this reaction is currently carried out via ozonolysis. While being a highly effective reaction, ozonolysis presents logistical and safety issues due to the production of highly reactive ozone and the explosive nature of the ozonide intermediates. Efforts to replicate the reactivity of ozone have, thus far, been unsuccessful and the closest reports describe oxidative cleavage of highly activated alkenes using molecular oxygen under photocatalytic conditions.
In search of ozone-type reactivity, Leonori and co-workers1 devised a strategy where nitroarenes could act as ozone surrogates in the cleavage of alkenes. As the nitro group is not as reactive as ozone, a thermal [3 + 2] cycloaddition is highly unlikely due to a large kinetic activation barrier. Excitation by visible light, however, gives a triplet state of the nitroarene which can undergo a stepwise addition to an alkene. Once the biradical intermediate recombines to give the “N-doped ozonide”, this intermediate is stable enough to isolate and the cleavage of this 5-membered ring can be carried out in a controlled manner to give the two aldehyde or ketone products.
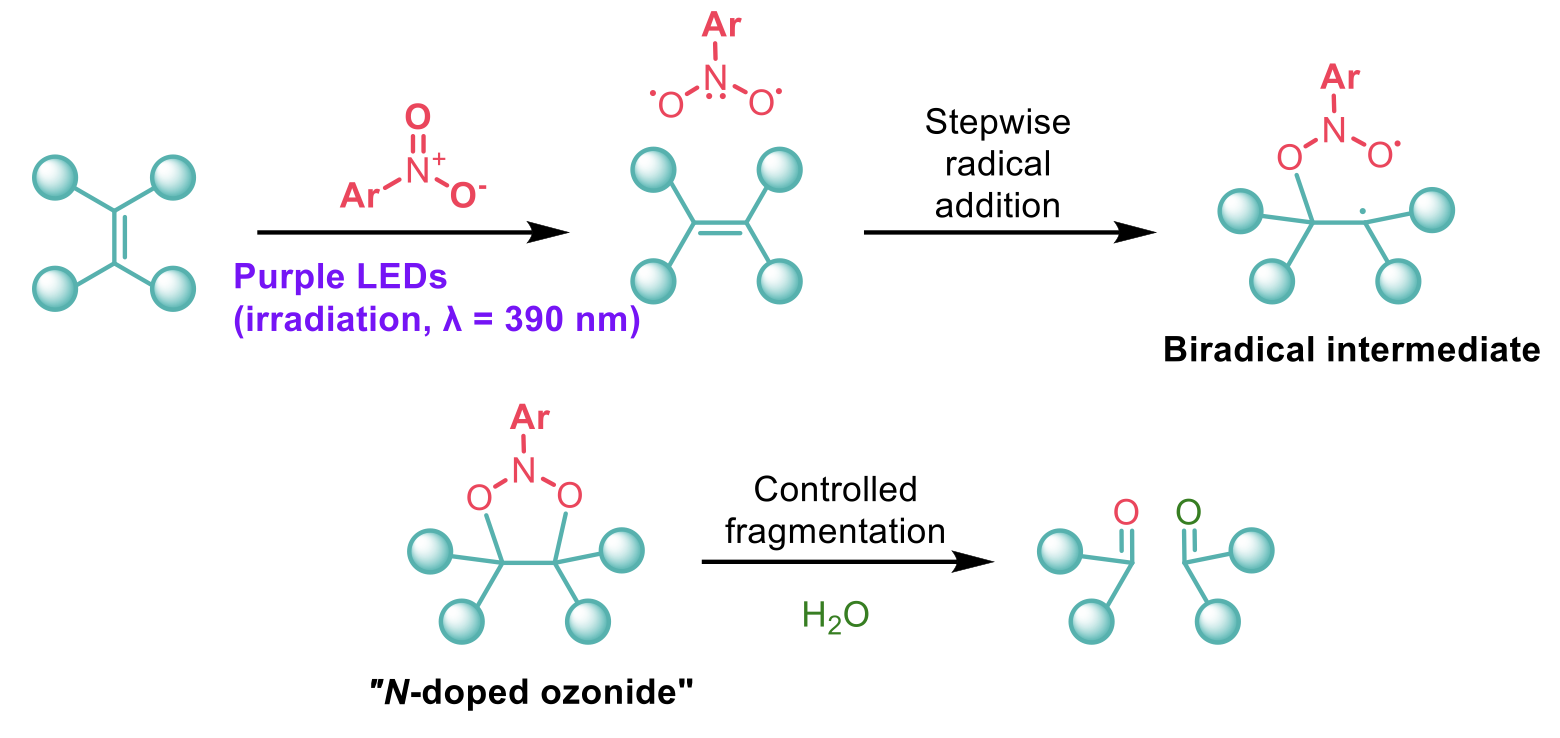
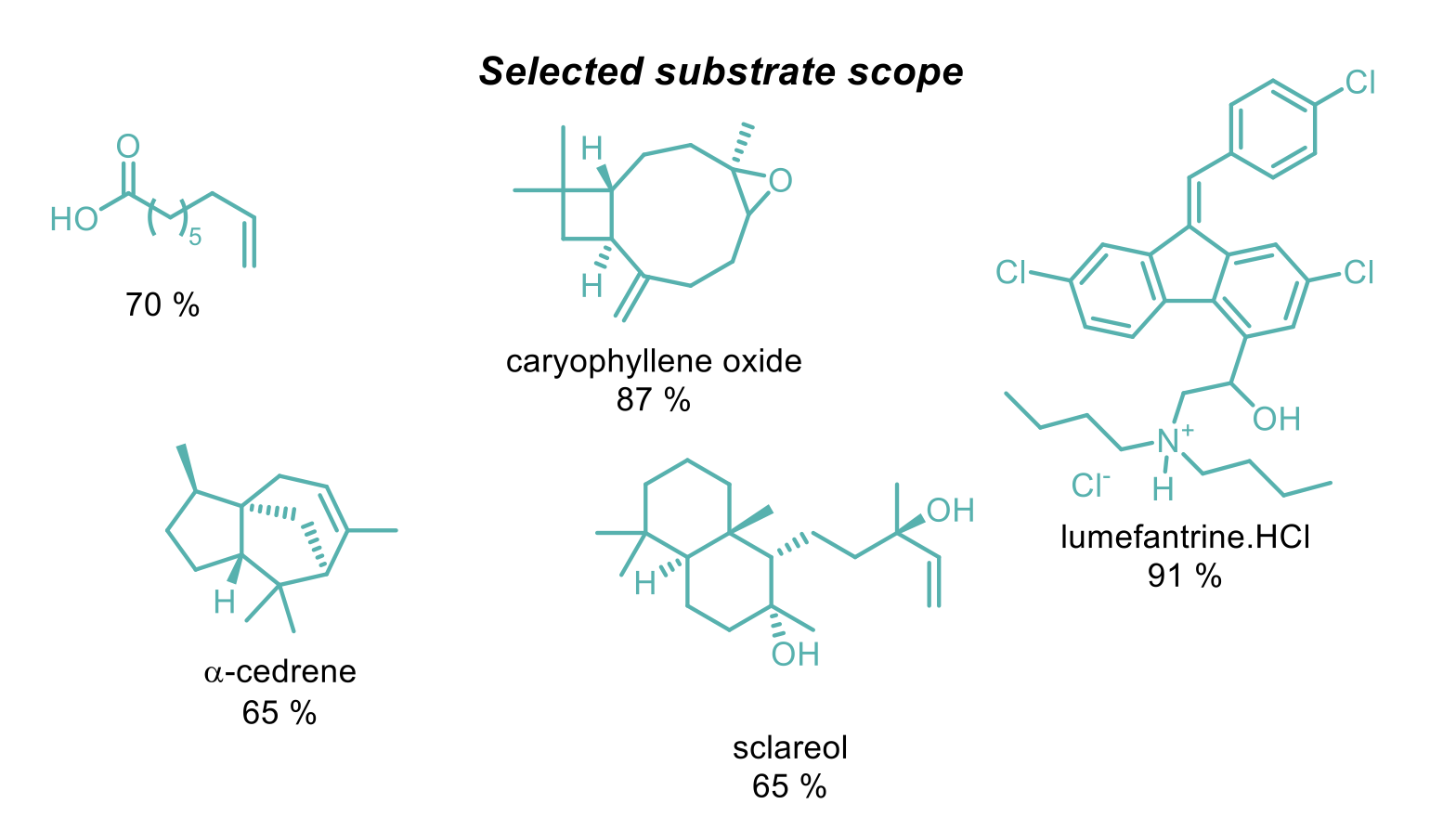
This transformation was demonstrated to be remarkably tolerant of functional groups including carbonyls, acids, halides, amines and alcohols (protected and unprotected), mono-, di-, tri-, and tetrasubstituted alkenes and a collection of pharmaceutically relevant substrates. Where more than one alkene was present in the molecule, the reaction is generally selective for the more electron-rich alkene and the selectivity can be increased by using a more electron-deficient nitroarene.
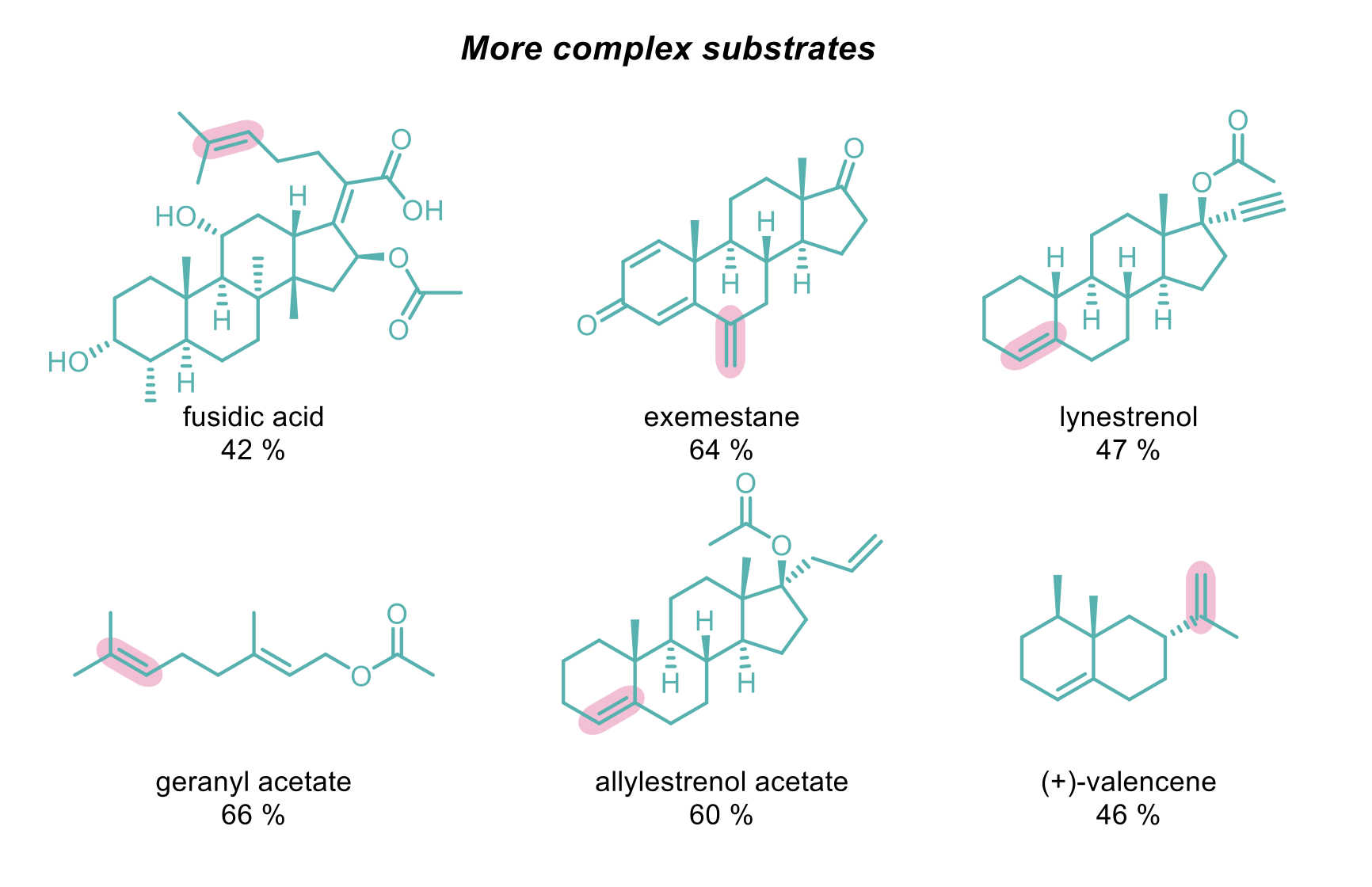
Demonstration of this reaction on steroids containing multiple alkenes was successful with selectivity generally preferred for the more electron-rich alkene, regardless of steric preferences. Only for one example was the selectivity-determining factor the steric constraints of the alkene, where the trisubstituted alkene with an α-tertiary centre was disfavoured over a 1,1-disubstituted alkene.
Brønsted acid-enhanced direct hydrogen atom transfer photocatalysis for selective functionalisation of unactivated C(sp3)-H bonds
Research into selective hydrogen-atom-transfer catalysis has, over the past decade, made great strides for activated C-H bonds but the cleavage of unactivated C-H bonds remains challenging. Although such methods are beginning to be reported, these suffer from low catalytic efficiency and are thus less appealing for late-stage modification reactions as the yield with respect to the C-H bond in question remains low.
To this end, Jie Wu and Xin Hong2 have reported a new method for the transformation of unactivated C-H bonds using a Bronsted-acid-enhanced hydrogen-atom-transfer photocatalyst. The excited photocatalyst can perform a direct hydrogen-atom abstraction from an alkane which is trapped by electron-deficient alkenes, pyridinium salts or Selectfluor to give alkylated, heteroarylated or fluorinated products, respectively.
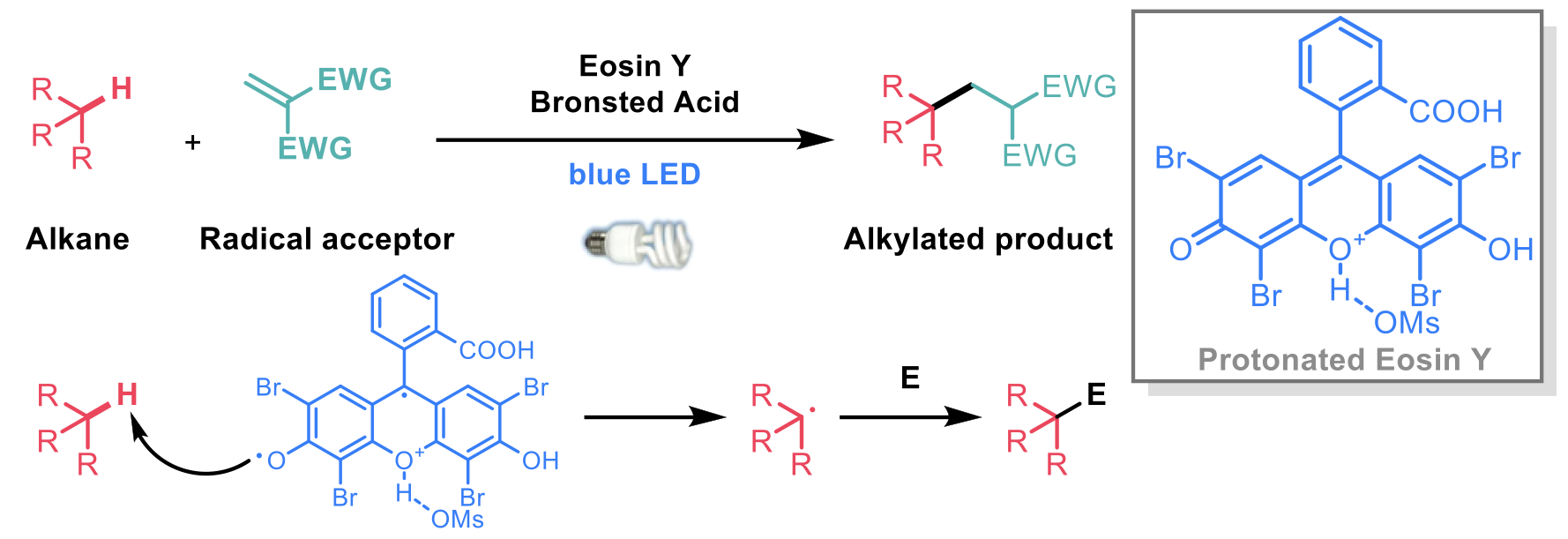
An inexpensive photocatalyst, Eosin Y, was observed to give low yields of alkylated product under standard conditions but addition of a Brønsted acid improved the efficiency by up to 88 % with respect to the alkane partner. A range of alkanes and electron-deficient alkenes were demonstrated to be suitable for this transformation and selectivity was observed for the most electron-rich C-H bond, usually tertiary. Using N-aminopyridinium salts as the acceptor, heteroarylation was also observed for a diverse collection of alkanes, both cyclic and acyclic. This protocol was also demonstrated to be suitable for fluorination chemistry with a similar range of substrates using Selectfluor as the radical acceptor.
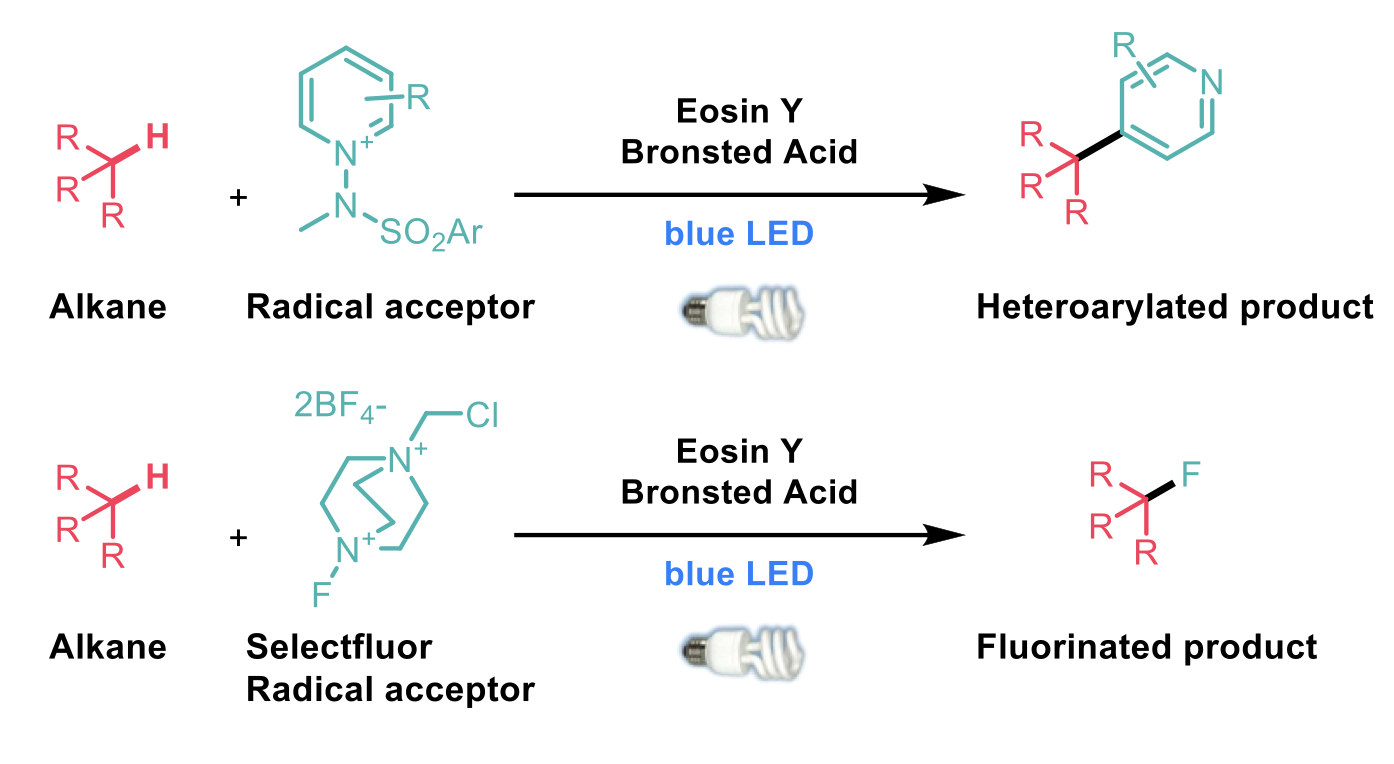
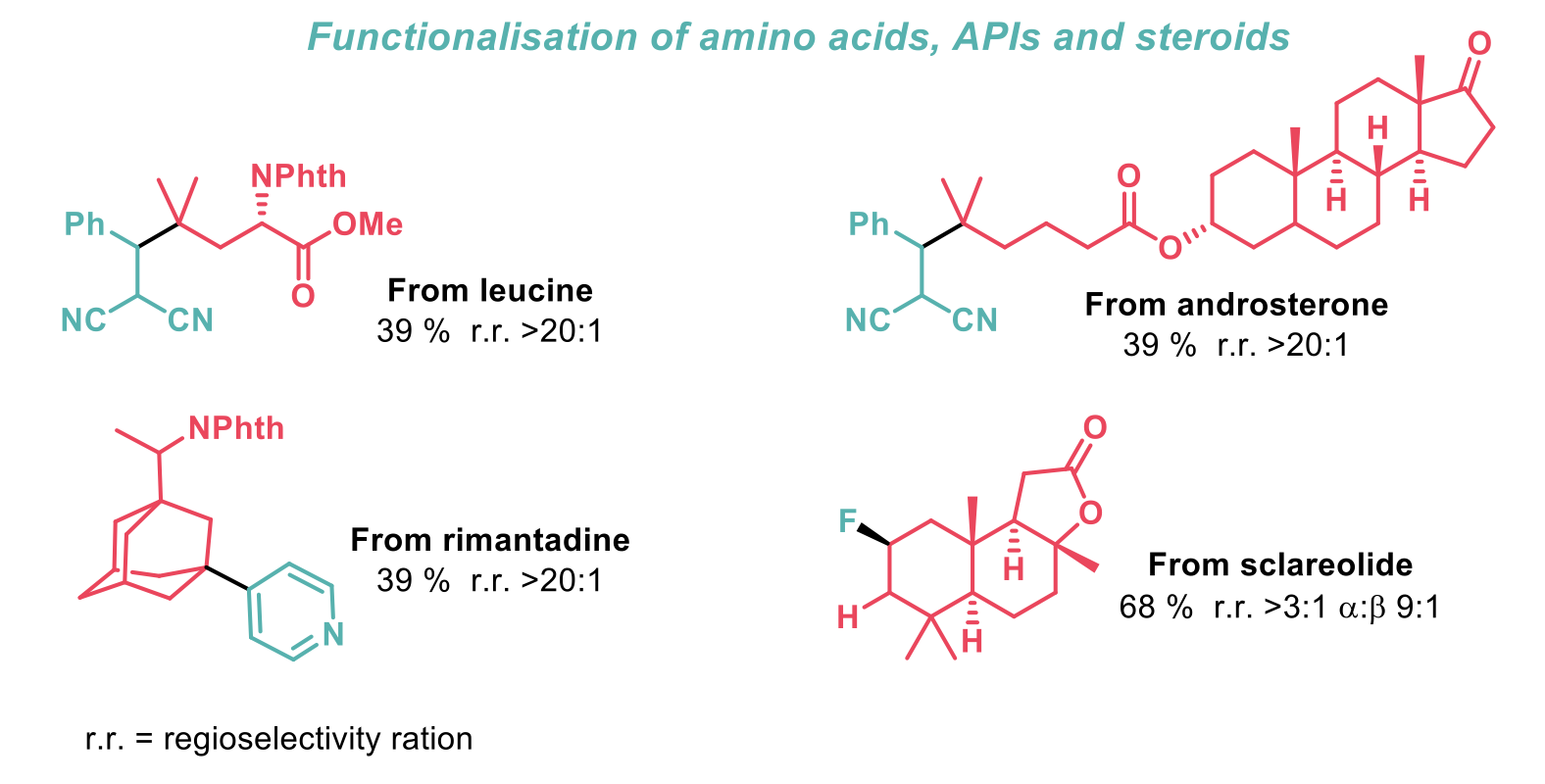
Late-stage functionalisation of pharmaceutically relevant molecules was illustrated in all three transformations, particularly with amino acids, adamantane containing APIs and steroids. Although the yields for some of these complex substrates decreased slightly, this represents a useful transformation for previously unreactive C-H bonds. The use of acid to increase the hydrogen-atom transfer ability of other photocatalysts was also investigated but this was only observed when an sp3 hybridised oxygen atom was present.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.