By Dr Alicia Galván Álvarez (Team Leader, Chemistry)
In this latest edition of our Medicinal Chemistry in Review blog series, our attention was drawn to a recent manuscript detailing the discovery of Elironrasib (RMC-6291), published by Revolution Medicines in the Journal of Medicinal Chemistry.1
RAS proteins have an important role in regulating normal cell growth and signaling. They act as molecular switches, cycling between an active, GTP-bound state or RAS(ON), and an inactive, GDP-bound state or RAS(OFF). Various well reported single point mutations in RAS proteins (G12, G13 and Q61) lead to increased RAS(ON) levels, causing excessive RAS signalling, resulting in uncontrolled cell growth and cancer progression.
While KRASG12C is a validated target in non-small cell lung cancer (NSCLC, ~15% of KRAS mutations), current inhibitor strategy has largely focussed only on the inactive, GDP-bound KRAS(OFF) state — leaving the active, GTP-bound form untouched. Targeting the active KRAS(ON) state through traditional small molecule drug discovery strategies has proven challenging as, for example, GTP or GDP occupy the only region of KRAS(ON) resembling a druggable binding pocket and the remaining surface is somewhat featureless, making competitive inhibition difficult.
The discovery of Elironrasib (RMC-6291) represents a significant breakthrough in targeting the previously undruggable active KRASG12C. Revolution Medicines has developed a new inhibitor modality involving formation of a tri-complex in which a small molecule inhibitor binds to the intracellular chaperone protein CypA, and this binary complex then interacts with the target protein KRASG12C(ON) via new complementary interactions, wherein the small molecule can covalently bind to C12 (Figure 1).
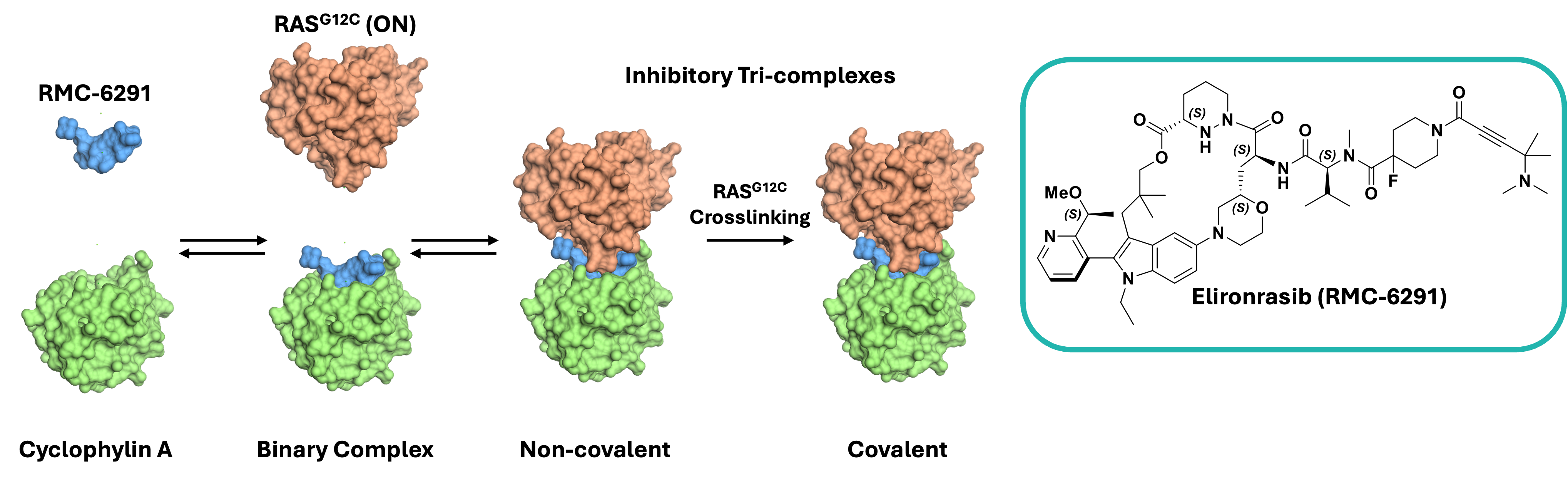
Figure 1. Schematic mechanism of tri-complex formation and Elironrasib (RMC-6291). Generated from PDB: 9BFX
After macrocyclisation of an early hit afforded compound 5, structure-guided medicinal chemistry efforts led the team to Elironrasib (Figure 2) via some key advancements:
- Optimisation of the left-hand side biaryl system and subsequent stabilisation of a single atropisomer (purple).
- Removal of the phenol group in the central linker of the molecule that limited both permeability and oral bioavailability (green).
- Identification of a novel, metabolically stable ynamide covalent warhead that increased KRASG12C cross-linking while reducing glutathione reactivity in parallel (yellow).
- Addition of a fluorinated linker on the right-hand side of the molecule that improved both oral bioavailability and on-target biological activity (blue).
- Substitution of the phenyl ring for morpholine which improved the degree of saturation (Fsp3) without increasing EPSA, which resulted in enhanced solubility and oral bioavailability (red).
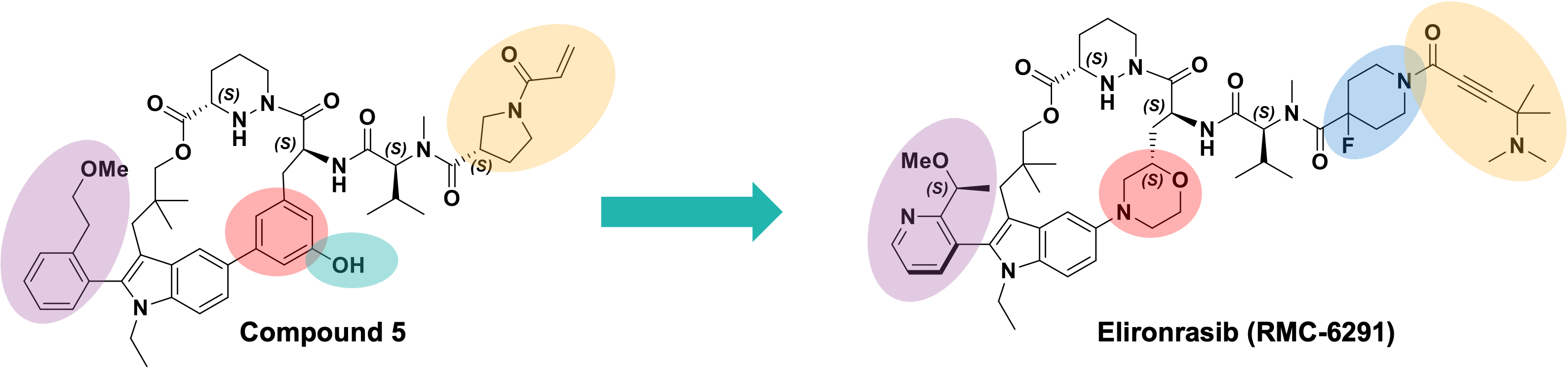
Figure 2. Development of Elironrasib from Compound 5
The resulting covalent tri-complex inhibits oncogenic signaling, inducing tumor regression across various preclinical models of KRASG12C mutant human cancers. This potent and selective, orally bioavailable, covalent tricomplex inhibitor is currently undergoing Phase 1 clinical trials (NCT05462717, NCT06128551, NCT06162221), with preliminary data showing promising results in patients resistant to first-generation KRASG12C inhibitors. Thus, positioning RMC-6291 as a potential next-generation therapy.
At Domainex, we have experience in the design, synthesis and analysis of covalent inhibitors and we offer a wide range of assays (including Kinact / KI assay for irreversible covalent compounds and an analogous assay format for reversible covalent inhibitors and GSH reactivity assessments), techniques and platforms to support both irreversible and reversible covalent inhibitor programmes from hit identification (including covalent fragment screening) through to hit-to-lead and lead optimisation. Please get in touch if you would like to find out more.
We hope you’ve found this interesting, if so look out for our next Medicinal Chemistry in Review blog, which will be available on our website soon.
Reference:
- Discovery of Elironrasib (RMC-6291), a Potent and Orally Bioavailable, RAS(ON) G12C-Selective, Covalent Tricomplex Inhibitor for the Treatment of Patients with RAS G12C-Addicted Cancers. James Cregg, Kristof Pota, Aidan C. A. Tomlinson, Jason Yano, Abby Marquez, Yang Liu, Christopher J. Schulze, Kyle J. Seamon, Matthew Holderfield, Xing Wei, Yongxian Zhuang, Yu Chi Yang, Jingjing Jiang, Yue Huang, Ruiping Zhao, Yun Ling, Zhican Wang, Michael Flagella, Zhengping Wang, Mallika Singh, John E. Knox, Robert Nichols, David Wildes, Jacqueline A. M. Smith, Elena S. Koltun, and Adrian L. Gill*. J. Med. Chem. 2025, 68, 6041−6063