- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Microsomal Clearance/Stability Assay
Metabolism is a major clearance mechanism that predominantly occurs in the liver. Liver microsomes are a subcellular fraction of the liver that can be derived from a variety of animals and used to determine the half-life (t1/2) or apparent in vitro intrinsic clearance (CLint, app) of test compounds. They contain cytochrome P450 enzymes, which are key Phase I drug-metabolising enzymes. Microsomal stability is often used in early lead-optimisation, due to the relatively low costs and high-throughput capability. Measuring microsomal clearance can not only assist in ranking/triaging compounds for in vivo studies, but also help to predict in vivo hepatic clearance.
Alternatively, liver S9 fractions or hepatocytes can be used to assess hepatic compound stability.
Domainex’s Standard Experimental Procedure:
Test compounds are incubated with liver microsomes in the presence of NADPH at 37°C. The reaction mixture is sampled at allocated timepoints into a cold stop plate containing acetonitrile and an internal standard. The samples are subsequently analysed by Ultra-High Performance Liquid Chromatography (UHPLC)-mass spectrometry (MS) to assess depletion of the test compound. In each experiment, control compounds with established CLint,app values are analysed alongside test compounds to confirm that microsomal enzyme activity is within the expected range. Example microsomal stability data is shown in Figures 1 and 2.
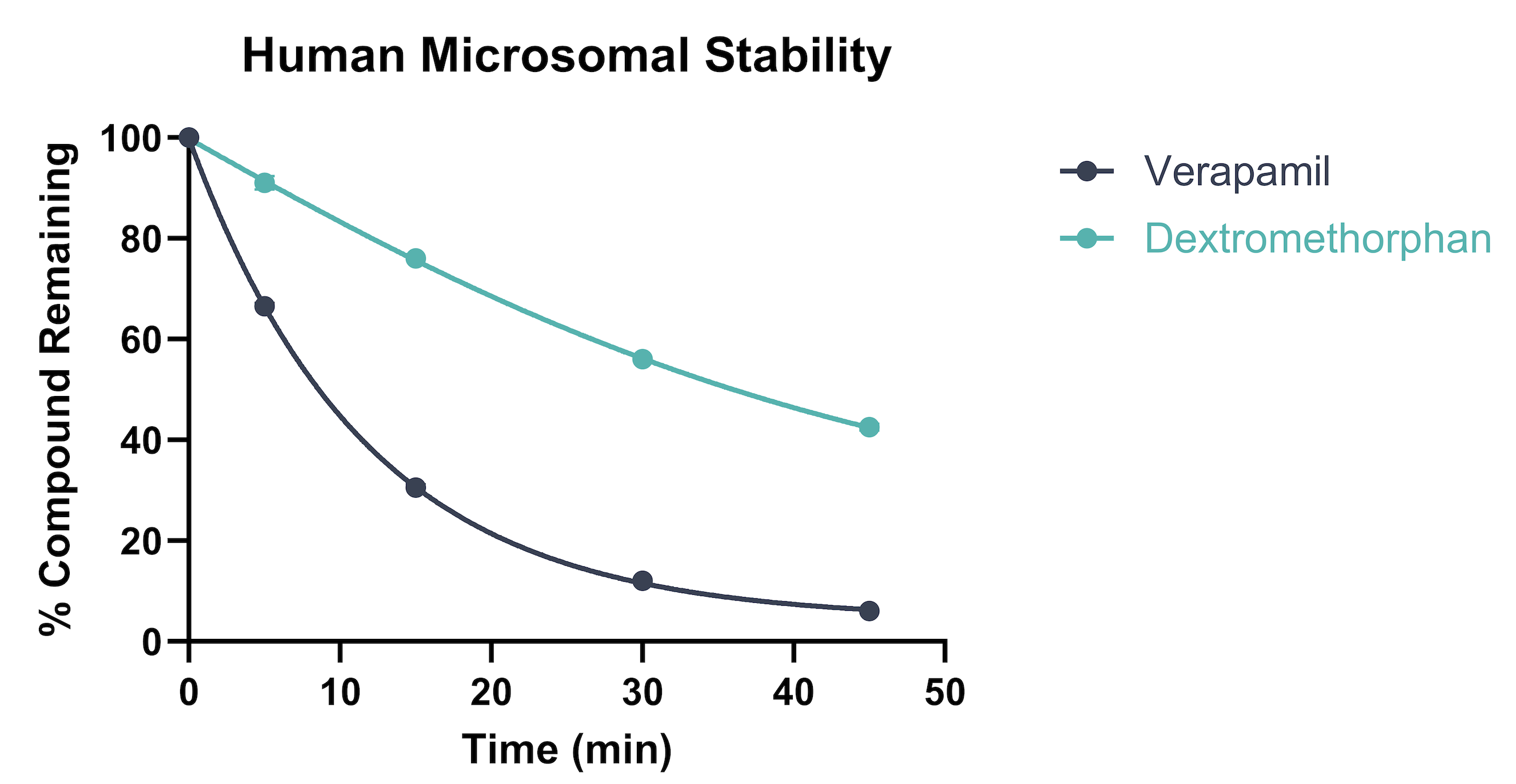
Figure 1. Example data showing depletion of test compounds following incubation with human liver microsomes (HLM).
* Other options available upon request
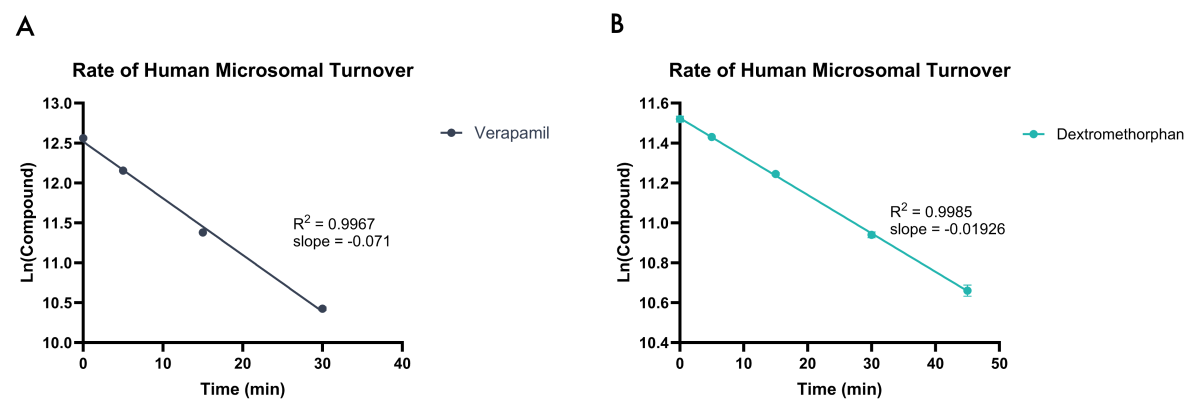
Figure 2. Example data showing rate of compound turnover in human liver microsomes for Verapamil (A) and Dextromethorphan (B). Natural Log linear plots of compound turnover allow CLint,app and t1/2 values to be calculated.
Data Analysis:
Microsomal incubation extracts are analysed using Waters Acquity UHPLC TQ-S, TQS-micro or TQ-XS instruments. Triple quadrupole mass spectrometers operated in multiple reaction monitoring (MRM) mode, provide accurate measurement with excellent sensitivity, selectivity and reproducibility.
Plotting the natural logarithm (ln) of compound response against time allows the determination of half-life (t1/2) and apparent intrinsic clearance (CLint, app) using the equations below:
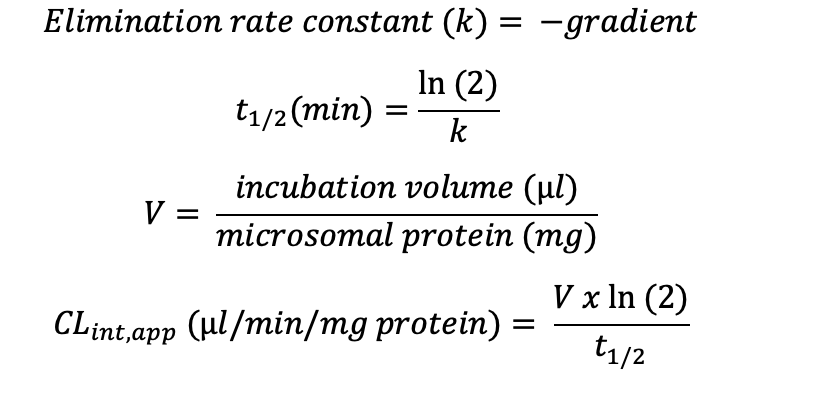
Deliverables:
The results are reported in Excel file format as CLint, app (µl/min/mg protein), t1/2 (min) and parent remaining (%) at T = 45 min in the presence and absence of NADPH, including standard error of mean (SEM). Any relevant comments about compound stability/solubility/binding are also included in the report.
Typical turnaround time from receipt of test compounds to release of data is ten working days or less.
Start your next project with Domainex
Contact one of our experts today