- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- DMPK
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Prodrugs
Overcoming obstacles by appending a promoiety to your drug
Approximately ten percent1 of all pharmaceutical agents are prodrugs, and at least thirty have reached the market since 2008.2 Despite their commercial success, prodrugs have historically been perceived as a last resort in the drug development process, however this is no longer the case, and between the mid-1990s and early 2000s there was a dramatic increase in the number of patents that mention them.3 Deciding to investigate prodrugs earlier in the drug discovery cycle may help overcome various medicinal chemistry challenges or offer vital additional patent protection. Initial prodrug assessments are relatively low-cost and can yield valuable insights in drug discovery programmes.
Prodrugs are compounds, that once administered, are cleaved, typically via an enzyme-mediated process, to release parent drug and a promoiety (Figure 1). This usually occurs in tissue such as liver or the intestine; but may also occur within plasma (typically esterase mediated). A rapid release profile is often preferred, although Domainex is adept at designing prodrugs with various release profiles and can tailor release rates to suit project needs.
Prodrugs are commonly used to overcome poor ADME properties, especially low permeability. For example, promoieties targeting peptide transporters (e.g., LAT1/2, PEPT1/2) can enhance uptake via transporter-mediated mechanisms. Other potential benefits include avoiding gut metabolism, reducing first-pass effects, mitigating toxicity, or improving patient compliance (e.g. masking unpleasant taste).
Modifying the number of hydrogen bond donors or adjusting lipophilicity through promoiety design and appendage can improve membrane permeability. At Domainex, we use Caco-2 or MDCK assays to assess compound permeability. ChromLogD data is obtained for every compound synthesised and we use these two key pieces of information for the next iteration of prodrug design.
Low aqueous solubility may limit systemic exposure and promoieties can be tailored to improve this physicochemical property, which can be assessed in-house via our kinetic or thermodynamic solubility assays in a range of media including Fasted State Simulated Intestinal Fluid (FaSSIF), Fed State Simulated Intestinal Fluid (FeSSIF), and other gastrointestinal-type fluids.
Various functional groups can be hydrolysed in an enzyme-mediated manner. Domainex can explore a variety of these to optimise hydrolysis rates across chemotypes. Our medicinal and computational chemists are expert molecular designers and can examine your compound(s) in detail to provide creative solutions. Neighbouring group participation can lead to faster release profiles, and we can investigate the effect of this strategy in parallel with different cleavable functional groups to vary the rate of drug release. Our bioanalytical team can assess the rate of turnover using in vitro and ex vivo assays to inform iterative design. The prodrug strategies employed will be determined by the functional groups that are exploitable within your parent molecule (for example amines, alcohols and carboxylic acids) and by the requirements of your project.
Domainex has extensive experience of evaluating prodrugs in biological matrices such as plasma and corneal tissue (ex vivo,) and conduction tissue analysis post in vivo PK studies. Using sensitive LC-MS, we quantify prodrug loss and drug/promoiety formation. Performing assays at different concentrations of compound can pinpoint whether hydrolysis is enzyme mediated. A representative hydrolysis profile is provided in Figure 2.
Further profiling in microsomes, hepatocytes or S9 fractions builds a comprehensive metabolic picture. We can also quantify intracellular concentrations to assess uptake and hydrolysis over time.
Finally, it is essential to confirm that the promoiety does not impair the drug’s biological activity. Our assay biologists develop and run a wide range of biochemical, biophysical and cellular assays to ensure efficacy is retained.
References
- Pharmacological Reports, 65, 1-14, 2013
- Nature Reviews Drug Discovery, 17, 559-587, 2018
- Drug Discovery Today, 19, 79-87, 2014
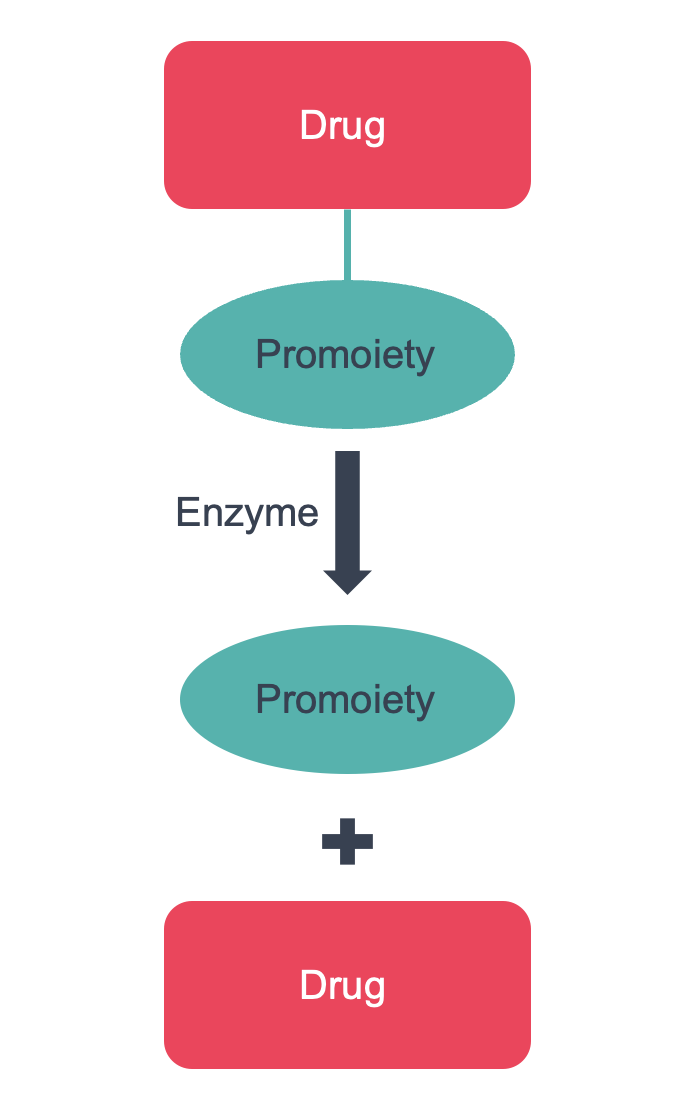
Figure 1: A prodrug comprises a drug (or potential drug) and a promoiety. The prodrug is cleaved in vivo (typically via an enzyme-mediated process), to release the parent drug and promoiety.
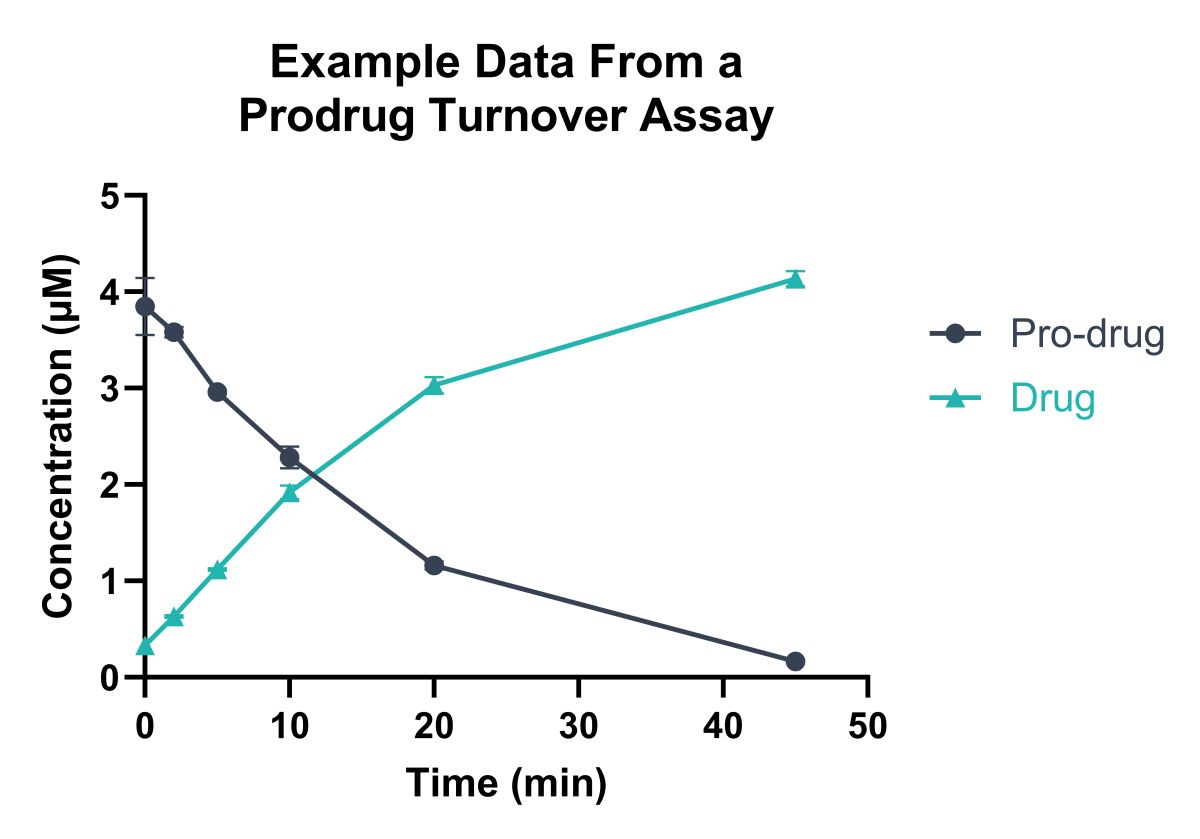
Figure 2: Example data from an ex-vivo tissue study, analysing total levels of pro-drug and drug. Compounds were monitored quantitatively over time by LC-MS/MS.
Start your next project with Domainex
Contact one of our experts today