By the Domainex Synthesis Group (Alicia Galván Álvarez, Claire Sear, Hugh Tawell, John May, Jonás Calleja Priede and Tenin Traore)
In the latest edition of our blog series, we have focused on the following two recent publications:
- Direct Deamination of Primary Amines via Isodiazene Intermediates. Kathleen J. Berger, Julia L. Driscoll, Mingbin Yuan, Balu D. Dherange, Osvaldo Gutierrez, and Mark D. Levin. J. Am. Chem. Soc. (2021)
- gem-Difluoroallylation of Aryl Halides and Pseudo Halides with Difluoroallylboron Reagents in High Regioselectivity. Shu Sakamoto, Trevor W. Butcher, Jonathan L. Yang, Prof. John F. Hartwig. Angew. Chem. Int. Ed. (2021)
Single Step and Effective Deamination of Primary Amines
While selective defunctionalizations of C−O, C−S, and C−X bonds have been employed strategically in organic synthesis, aliphatic deamination protocol remains elusive. Although aliphatic primary amines are ubiquitous in pharmaceuticals and natural products, their poor nucleofugality renders substitution chemistry highly challenging. The state-of-the-art for aliphatic deaminative substitution involves prefunctionalisation of primary amines to Katritzky-type pyridinium salts, which undergo photocatalytic or metal-catalyzed cross-coupling reactions. Otherwise the remaining literature for direct deamination uniformly requires harsh reagents or conditions, and/or suffers from low functional group tolerance and yield.
In this recent publication from Mark D. Levin et al.1, the authors report a simple method for the deamination of primary amines, using an anomeric amide, which proceeds in a single step under remarkably mild conditions and with exquisite functional group tolerance.
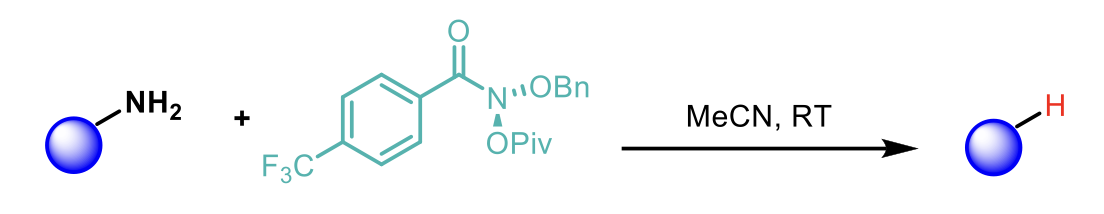
Previous work by the group, showed that the mechanism of the reaction for secondary amines involves N-pivaloyloxy-N-alkoxyamides (“anomeric amides”) that promote nitrogen deletion of secondary amines through a sequence involving nucleophilic substitution by the amine, followed by reductive elimination to form an isodiazene intermediate. Subsequent loss of dinitrogen yields a geminate radical pair which recombines principally within the primary solvent cage, resulting in the net “deletion” of the amine.
The authors hypothesised that anomeric amides would undergo an analogous substitution with primary amines to generate primary isodiazene intermediates (see below).
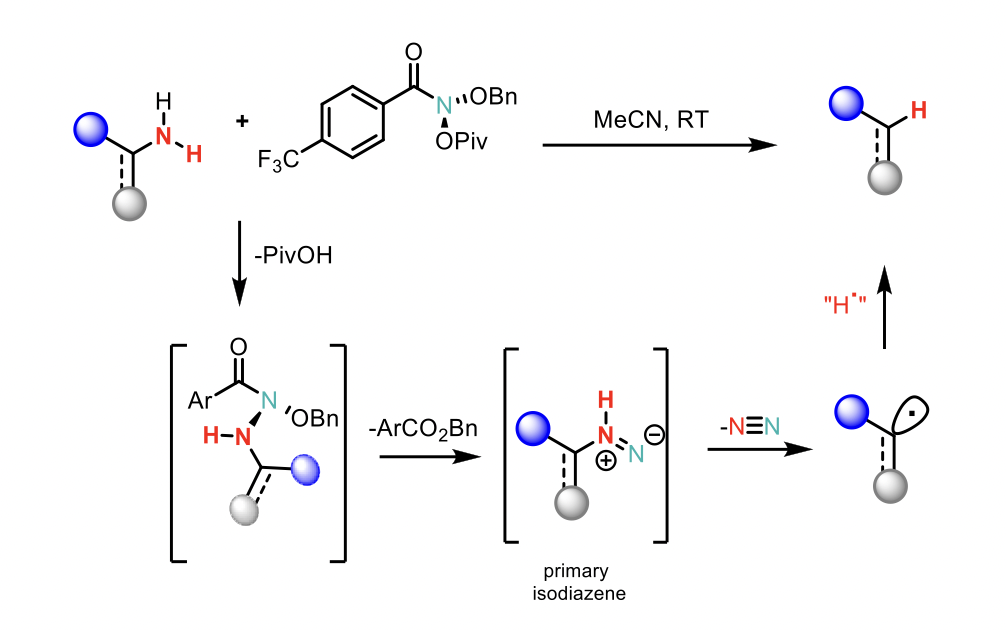
However, unlike secondary isodiazenes, which have been well-studied and even spectroscopically characterised at cryogenic temperatures, primary isodiazenes are almost entirely unprecedented, suggesting an opportunity to leverage a novel reactive intermediate.
This methodology was used for the elaboration of novel antibiotics and more specifically for the synthesis and preliminary biological evaluation of 3′-substituted cephem sulfones as potential β-Lactamase inhibitors.
Highly Regioselective gem-Difluoroallylation of Aryl halides and Pseudo Halides with Difluoroallylboron Reagents
The difluoromethylene (CF2) moiety has application in optimising chemical, biological and physical properties across a range of fields e.g., pharmaceuticals, materials, and agrochemicals. In this recent publication from John F. Hartwig et al.2 at the University of California, a palladium-catalysed gem-difluoroallylation of aryl halides and pseudo halides with high regioselectivity and in high yield is reported.
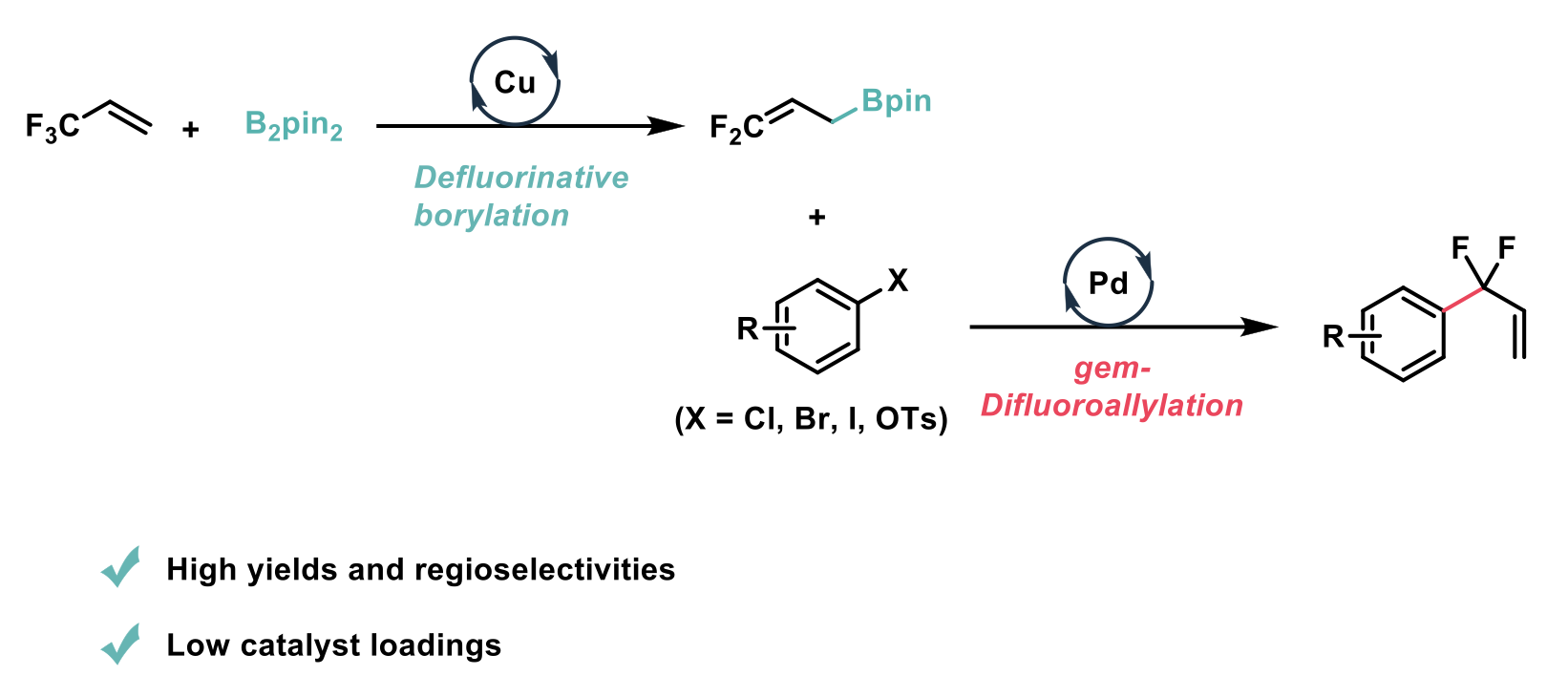
This method improves on previously reported methods as it employs the affordable 3,3,3-trifluoropropene to access the difluoroallyl boronates. A singular example of a previous cross-coupling of an aryl halide with a substituted 3,3-difluoroallyl boronate existed in the literature, however it had only a moderate yield, required a high catalyst loading (10 mol%) and necessitated that iodide was the leaving group on the arene.
This work reports the successful palladium-catalysed gem-difluoroallyation of aryl bromides, chlorides, iodides and triflates. The reactions require a low catalyst loading (0.1-2.5 mol% [Pd]) and the reactions proceed in high yields with high regioselectivities. To achieve this goal, 3,3-difluoroallyl pinacol boronate was accessed from the inexpensive 3,3,3-trifluoropropene. To perform the desired gem-difluoroallyation, the difluoroallyl boronate was reacted with selected aryl halides in the presence of Pd2(dba)3, cataCXium PICy, and utilising K2CO3 as the base.
The broad scope of this chemistry means that this methodology will be of high value in the field of medicinal chemistry as a means to access novel fluorinated building blocks.
We hope you found this interesting! Look out for our next synthesis review which will be available on our website soon, and get in touch to find out how we can employ the latest synthetic approaches to solve your drug discovery challenges.