By the Domainex Synthesis Group (Alicia Galván Álvarez, Hugh Tawell, Tenin Traore, Andrew Jones, Jonas Calleja, David Gibson, Andrea Bombana, James Sharpe, Venu Komanduri, Fergus Preston, Brahmam Medapi and Robyn Presland)
In the latest edition of our blog series, we have focused on the following three recent publications, which describe highly useful synthetic transformations:
- One-Pot Synthesis of Sulfonamides from Unactivated Acids and Amines via Aromatic Decarboxylative Halosulfonylation. David W. C. MacMillan et. al., J. Am. Chem. Soc., 2023, 145, 39, 21189-21196.
- Photocatalytic Carboxylate to Sulfinamide Switching Delivers a Divergent Synthesis of Sulfonamides and Sulfonimidamides. Michael C. Willis et. al., J. Am. Chem. Soc., 2023, 145, 39, 21623-21623.
- Photoredox Catalysis-Enabled Sulfination of Alcohols and Bromides. W P. Carson II, P J. Sarver, N S Goudy, and D W. C. Macmillan., J. Am. Chem. Soc. 2023, 145, 38, 20767-20774.
Shedding Light on Sulfonamide Synthesis: Photocatalytic Decarboxylative Approaches
One-pot synthesis of sulfonamides from simple readily available and structurally diverse aryl and alkyl carboxylic acids via photolytic decarboxylation using different catalysts has gained traction in recent years due to their high demand in medicinal chemistry. The reason for this demand for sulfonamides is that they are amide bioisosteres which possess potentially advantageous features, such as an additional hydrogen bond acceptor, hydrolytic stability, increased polar surface area (PSA), and improved binding properties.
MacMillan et al1 developed a methodology for accessing (hetero)aryl sulfonamides from (hetero)aryl carboxylic acids and amines by a copper catalysed (Cu(MeCN)4BF4) decarboxylative halosulfonylation in one-pot. They employed the Cu-ligand-to-metal charge transfer (LCMT) method for effective decarboxylative halosulfonylation via aryl radical engagement with SO2 to forge C(sp2)-S bonds and generate the aryl sulfonyl radical. The sulfonyl radical then reacts with electrophilic halogenating reagents (selectfluor, (DCDMH)) leading to the desired aryl sulfonyl halide. Subsequently reacting the halosulfonyl halides with amines in the presence of base results in the formation of the desired structurally diverse sulfonamide. The authors limited this method only to (hetero)aromatic acids. Alkyl and cycloalkyl variants were not mentioned.
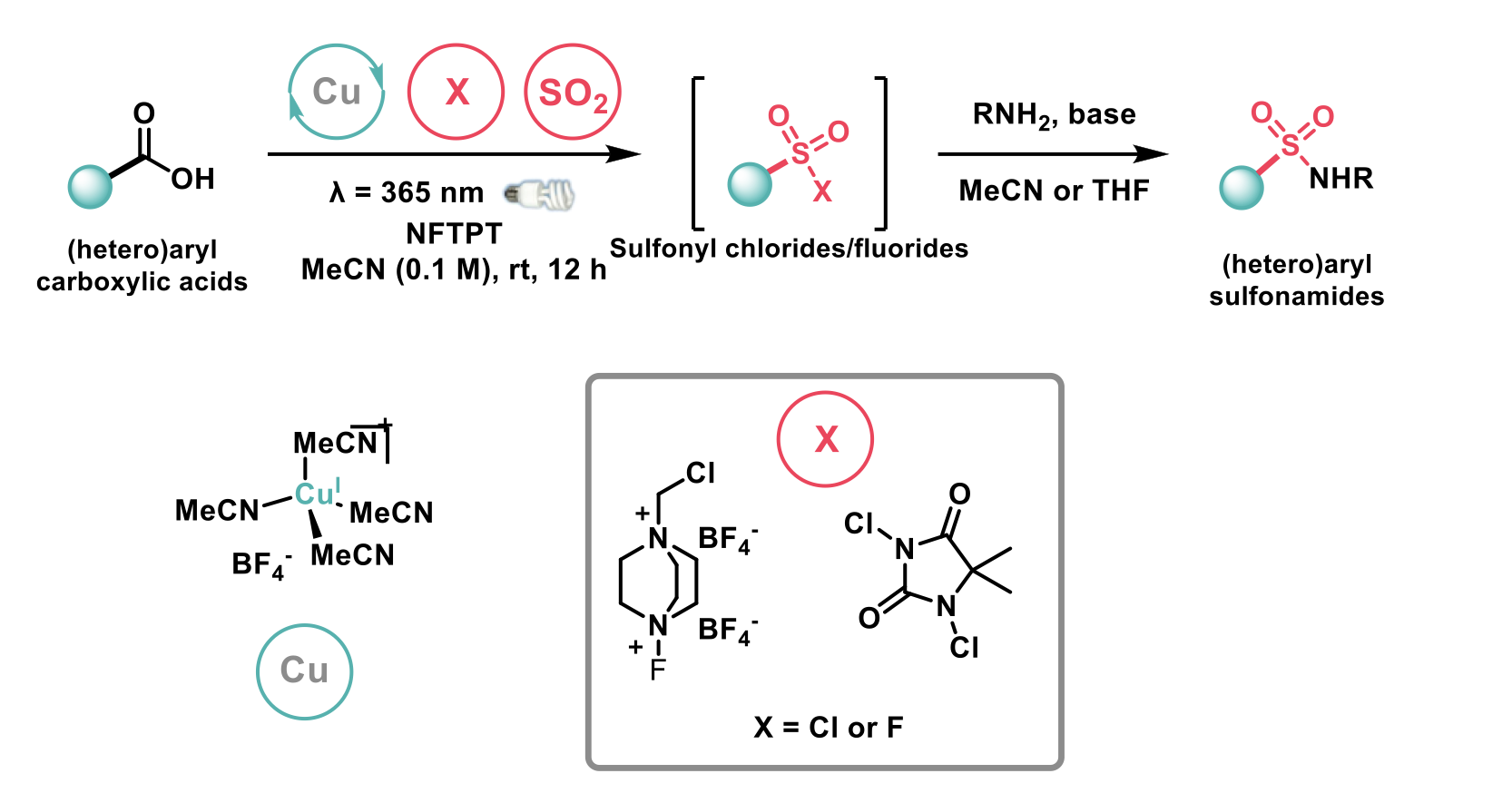
Furthermore, the authors demonstrated rapid access to amide bioisosteres and late-stage functionalisation of structurally diverse molecules with good yields.
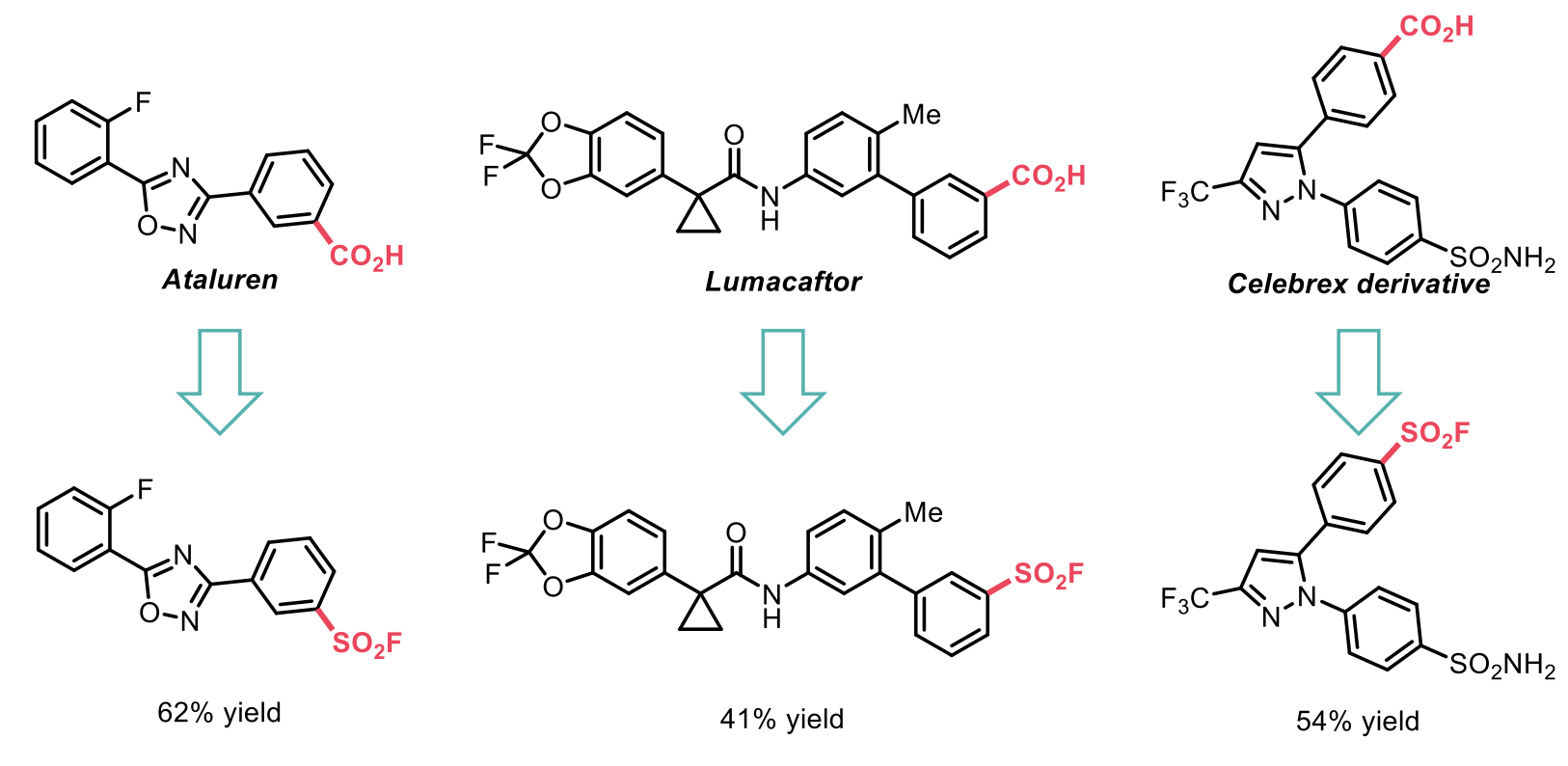
Despite the obvious advantages of a one-pot reaction system, the substrate scope of this work was limited to only (hetero)aryl carboxylic acids. Other authors have developed a method that is applicable to a different substrate scope, namely alkyl carboxylic acids that complements the former reaction well.
Work by Michael C. Willis et al.2 approached divergent methodology for accessing structurally distinct sulfonamides and sulfonimidamide from alkyl and cyclic (including spirocyclic) carboxylic acids via in situ generation of sulfinamide using an acridine based photocatalyst followed by reactions with different amines. The majority of the work focussed on the synthesis of stable sulfinamides via a decarboxylative approach with sulfinylamines. The authors demonstrated wide functional tolerance for the sulfinamide synthesis with this approach.
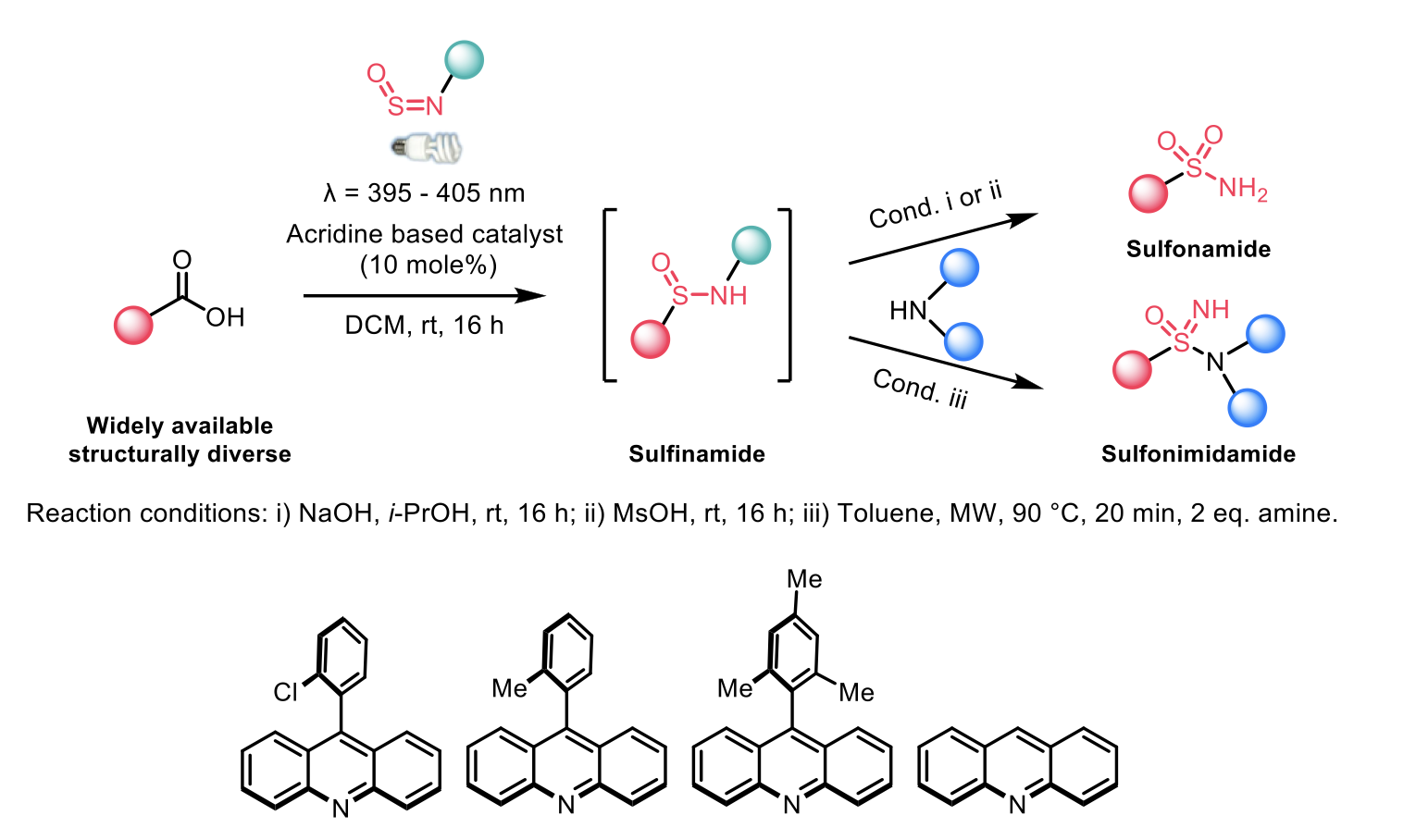
In conclusion, the two sets of authors have devised different strategies for the effective synthesis of structurally distinct sulfonamides, sulfinamide, and sulfonimidamides using different photocatalysts via photodecarboxylation, and have also elaborated the late-stage functionalisation of drugs and biologically significant molecules with moderate to good yields.
Photoredox Enables the Sulfination of Alcohols and Bromides
Sulfinates (R-SO2-) serve as important intermediates in pharmaceutical chemistry, as they can be utilised to access a wide range of important sulfur containing compounds. Synthesising this class of compound via photoredox chemistry has posed a challenge due to the ease of their oxidation. Previously, this issue of oxidation has been overcome by using net-reductive conditions, proton-coupled electron transfer (PCET) or hydrogen-atom transfer (HAT). The main limitation of these approaches is the restriction of functional groups that can be tolerated, limiting the scope of the transformation.
To this end, Macmillan et al.3 have reported a photocatalytic method for direct conversion of alcohols and alkyl bromides into alkyl sulfinates.
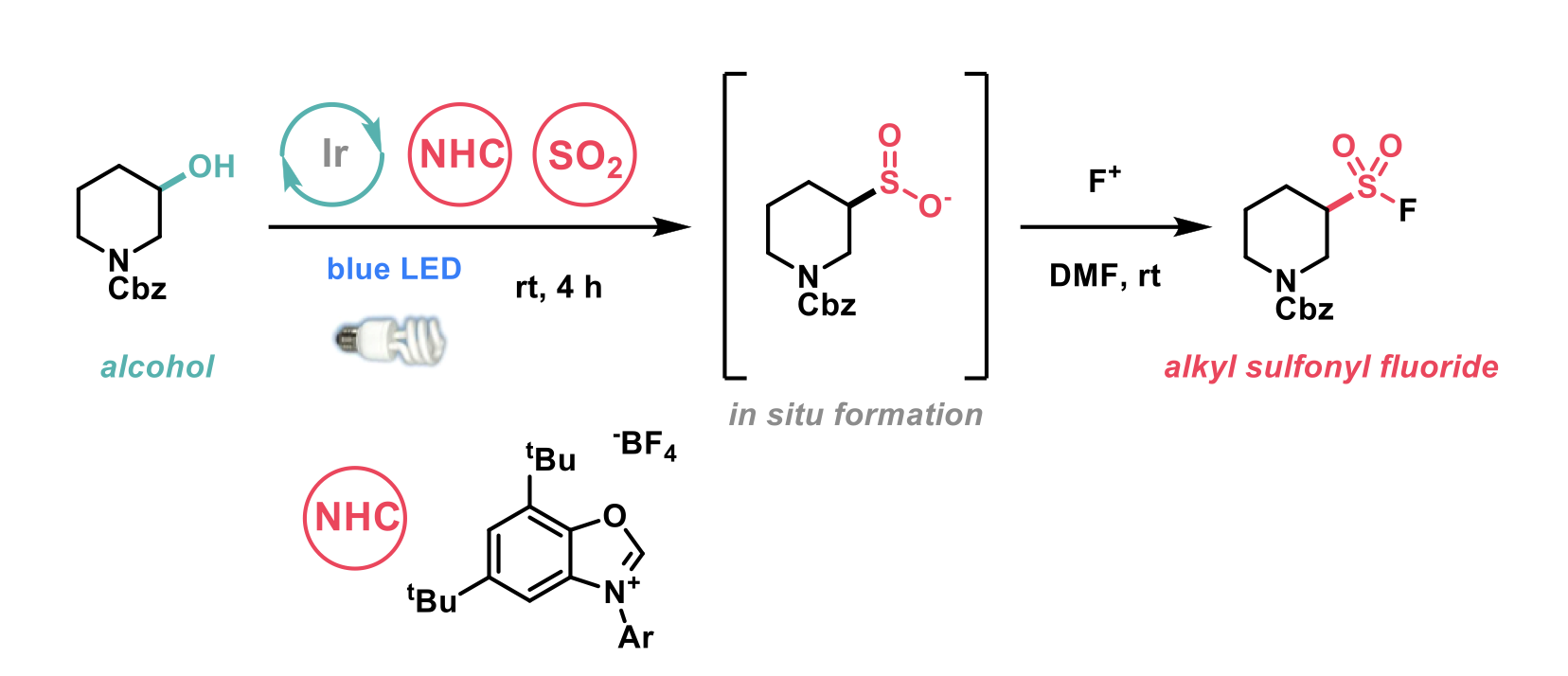
The group first considered the sulfination of alcohols due to their prevalence as a functional group. Their recently published work utilised an N-heterocyclic carbene-alcohol adduct (NHC-OR) for the radical deoxygenative arylation of alcohols. This activated NHC-OR adduct was found to have an oxidation potential much lower than alternative oxidative methods for generating alkyl radicals from alcohols. Furthermore, the group carried out Stern-Volmer quenching studies which demonstrated this adduct outcompetes cesium oxalates, sodium sulfates and zinc in photocatalyst quenching. These two features and that the NHC-alcohol adduct can be generated using mild conditions and without purification makes it a highly suitable intermediate in the desired transformation. In combination with a suitable SO2 source and a photocatalyst, it was hypothesised that this could be a broadly applicable method for performing the desirable transformation of an alcohol to the corresponding sulfinate.
In addition to alcohols the group turned their attention to alkyl bromides, another important functionality and readily available feedstock. Recently published work demonstrated the formation of an N-adamantyl aminosupersilane widely applied in the context of the cross-electrophile coupling of unactivated chlorides.4 Results of cyclic voltammetry and Stern-Volmer experiments were comparable to those observed in the case of the NHC-alcohol adduct i.e., lower oxidation potential and outcompeting sodium sulfinate and supersilanol in photocatalyst quenching.
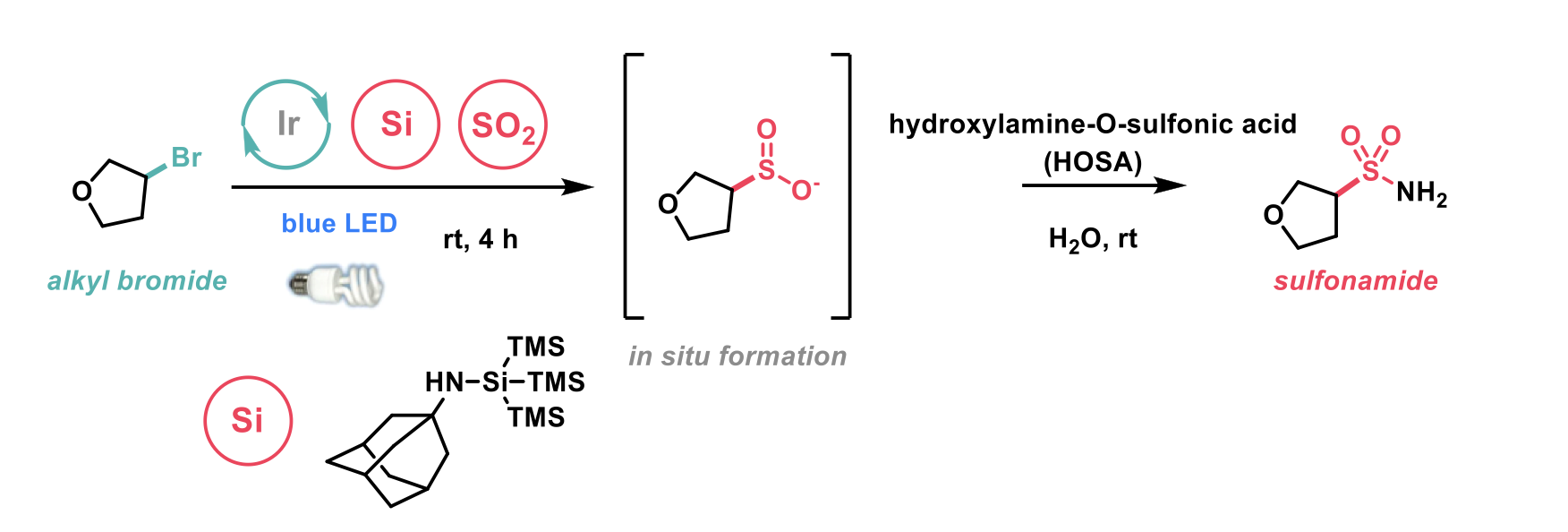
Following optimisation of the reaction conditions on a model alcohol substrate, which afforded the desired sulfinate in excellent isolated yields, the authors applied the conditions to a broad scope of substrates. After generating the sulfinate, Selectfluor was added to access sulfonyl fluorides. The optimised conditions afforded the desired transformation on a range of primary, secondary, and tertiary alcohols in good yields. Substrates that were well tolerated included unprotected phenols as well as aryl bromides, which the authors highlighted as an opportunity for further diversification. Saturated heterocycles were reported to undergo the transformation in good to excellent yields, spirocyclic motifs were also well tolerated. The substrate scope for the alkyl bromides was also broad with primary, secondary and tertiary alkyl bromides undergoing the transformation in good to excellent yields. In addition to the scope of alkyl alcohols and alkyl bromides the diversification of the sulfinate was also explored. A diverse range of sulfinates were accessed with this chemistry permitting the quick conversion of alcohols to medicinally relevant and desirable substituted sulfonamides.
Following demonstration of a broad substrate scope, the authors showed how their sulfination method could be incorporated into late-stage functionalisation of drugs and biologically relevant molecules in moderate yields.
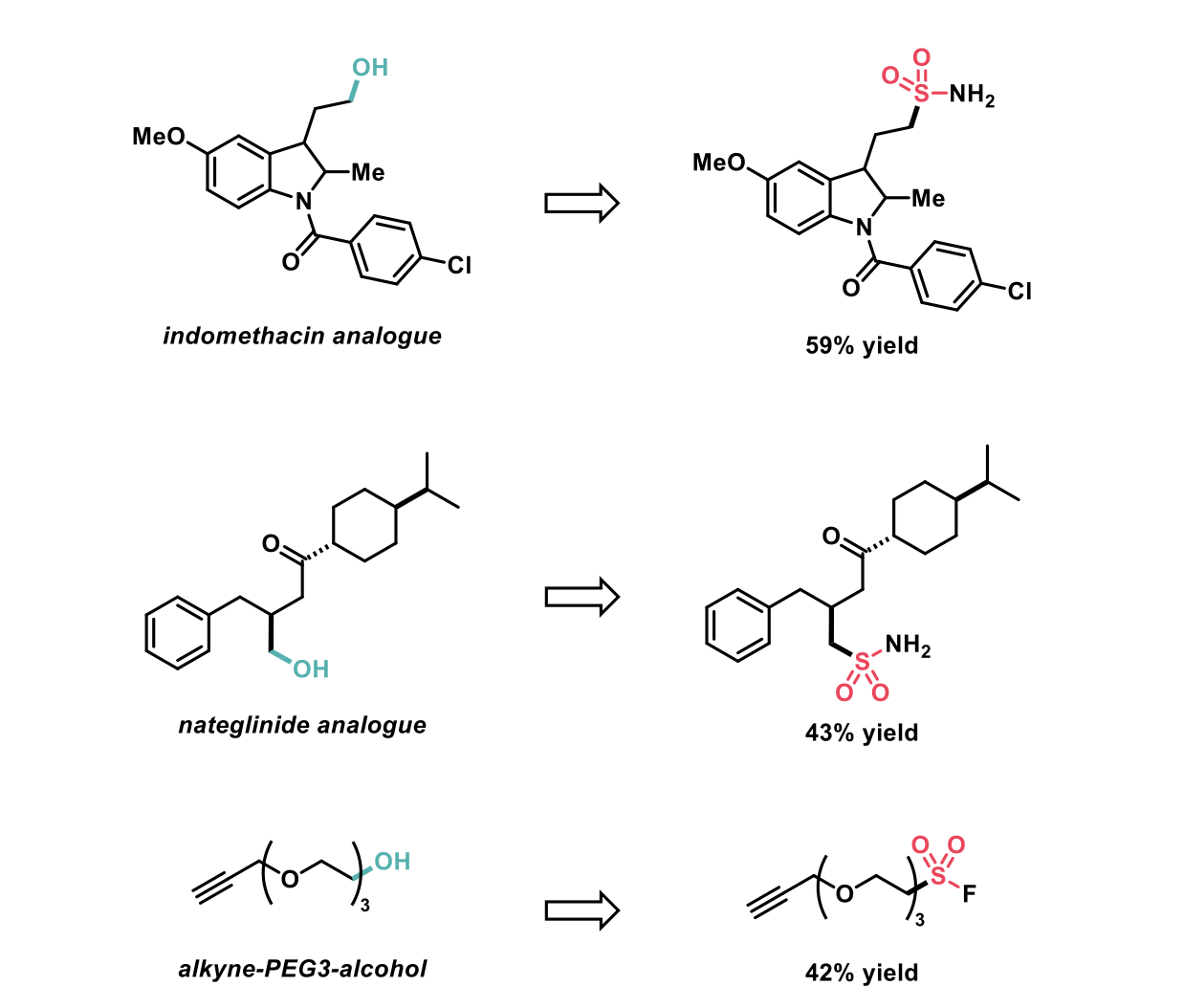
In conclusion this novel chemistry, utilising photoredox catalysis, provides general methods for debrominative and deoxygenative sulfination. These methods have large substrate scope, afford good/excellent yields as well as being applicable to biologically relevant molecules.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.
Additional reference:
4. Cross-Electrophile Coupling of Unactivated Alkyl Chlorides. Sakai, H. a.; Liu, W.; Le, C.; MacMillan, D. W. C., J. Am. Chem. Soc. 2020, 142, 27, 11691-11697.