By the Domainex Synthesis Group (Andrea Bombana, Hugh Tawell, Andrew Jones, Venu Komanduri, Brahmam Medapi, Robyn Presland, and Vinny Duong)
In this blog post, we delve into two recent publications that highlight innovative and impactful synthetic methodologies:
- Angular Spirocyclic Azetidines: Synthesis, Characterisation, and Evaluation in Drug Discovery. Alexander A. Kirichok, Hennadii Tkachuk, Kostiantyn Levchenko, Dmitry Granat, Tetyana Yegorova, Dmytro Lesyk, Anna Anisiforova, Yuliia Holota, Viktoriia Zomchak, Iryna Bodenchuk, Viktoriia Kosach, Petro Borysko, Rodion A. Korzh, Galeb Al-Maali, Vladimir Kubyshkin, Henry S. Rzepa, Pavel K. Mykhailiuk, Angew. Chem. Int. Ed., 2024.
- Light-promoted aromatic denitrative chlorination. Tiantian Liang, Zhen Lyu, Ye Wang, Wenyan Zhao, Ruocheng Sang, Gui-Juan Cheng, Fei Ye. Nat. Chem., 2025.
Angular Spirocyclic Azetidines: Synthesis, Characterisation, and Evaluation in Drug Discovery
“Linear” spirocyclic azetidines are known bioisosteres for saturated azaheterocycles such as piperidine, piperazine, and morpholine. However, the isomeric “angular” spirocyclic azetidines have been less explored as bioisosteres in part due to their more challenging synthesis. In this paper by Mykhailiuk et al1, alkenes were reacted with Graf’s isocyanate to yield β-lactams, which were subsequently reduced with aluminium hydride to yield “angular” spirocyclic azetidines (Scheme 1).
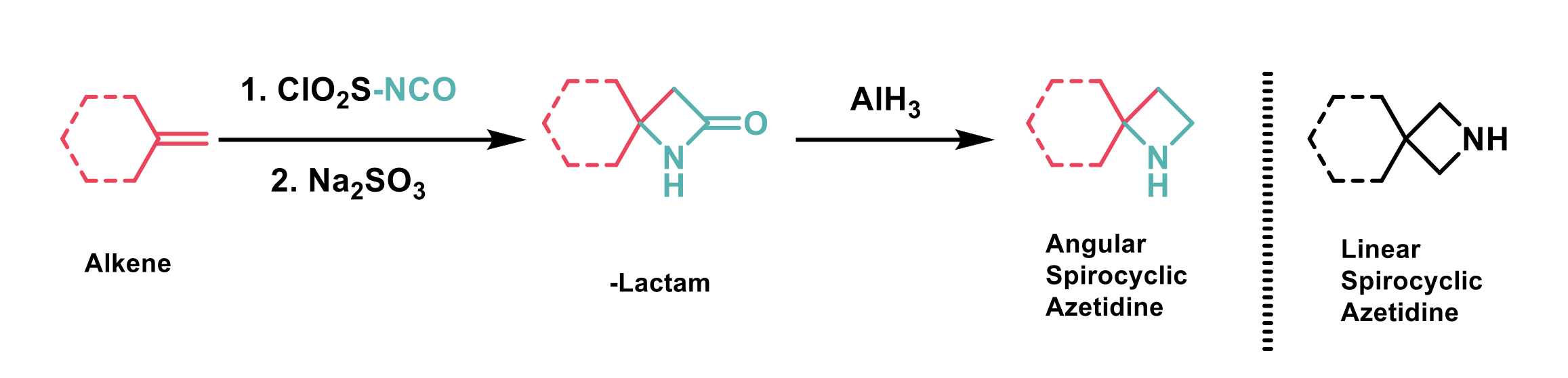
Scheme 1: Synthesis of angular spirocyclic azetidines.
Alkenes containing five, six, and seven membered rings successfully underwent reaction with Graf’s isocyanate to yield the corresponding β-lactams and it was found that CF3, F, ether, ester, and N-Cbz functional groups were also compatible. The diastereoselectivity of the reaction of chiral substrates was found to be substrate dependent, substrates containing alkyl substituents gave the cis product, whereas substrates containing ester substituents gave the trans product. Unfortunately, alkenes containing active α-methylene groups or sulfur were incompatible with the reaction conditions. The reaction worked well on large scale, and was successfully performed on 68 g of alkene in one case. The authors demonstrated that the “angular” azetidine products could undergo a range of transformations to yield a variety of functionalised “angular” azetidine products.
It was found that the replacement of six-membered heterocycles (morpholine and piperazine) with the corresponding azetidines resulted in an increase in nitrogen basicity, this was more pronounced in the “linear” azetidines than in the “angular” azetidines. It was also demonstrated that “angular” azetidines occupy different regions of chemical space than piperazine as their vector characteristics vary significantly.
Azetidine analogues of Sonidegib (an anticancer drug) and Danofloxacin (an antibacterial drug) were prepared. It was found that the effect of replacing saturated heterocyclic rings with azetidine analogues on water solubility was substrate dependent. In some examples water solubility increased and in other examples it decreased. The replacement of morpholine/piperazine with “angular” azetidines either reduced the lipophilicity or did not affect it. Finally, it was found that the “angular” isomer of Sonidegib, 66 was slightly less active in Gli-Luc reporter NIH3T3 cells, with an IC50 value of 21 nM compared to 6 nM for Sonidegib, and was more active than the “linear” analogue, 65, which had an IC50 value of 116 nM (Figure 1).
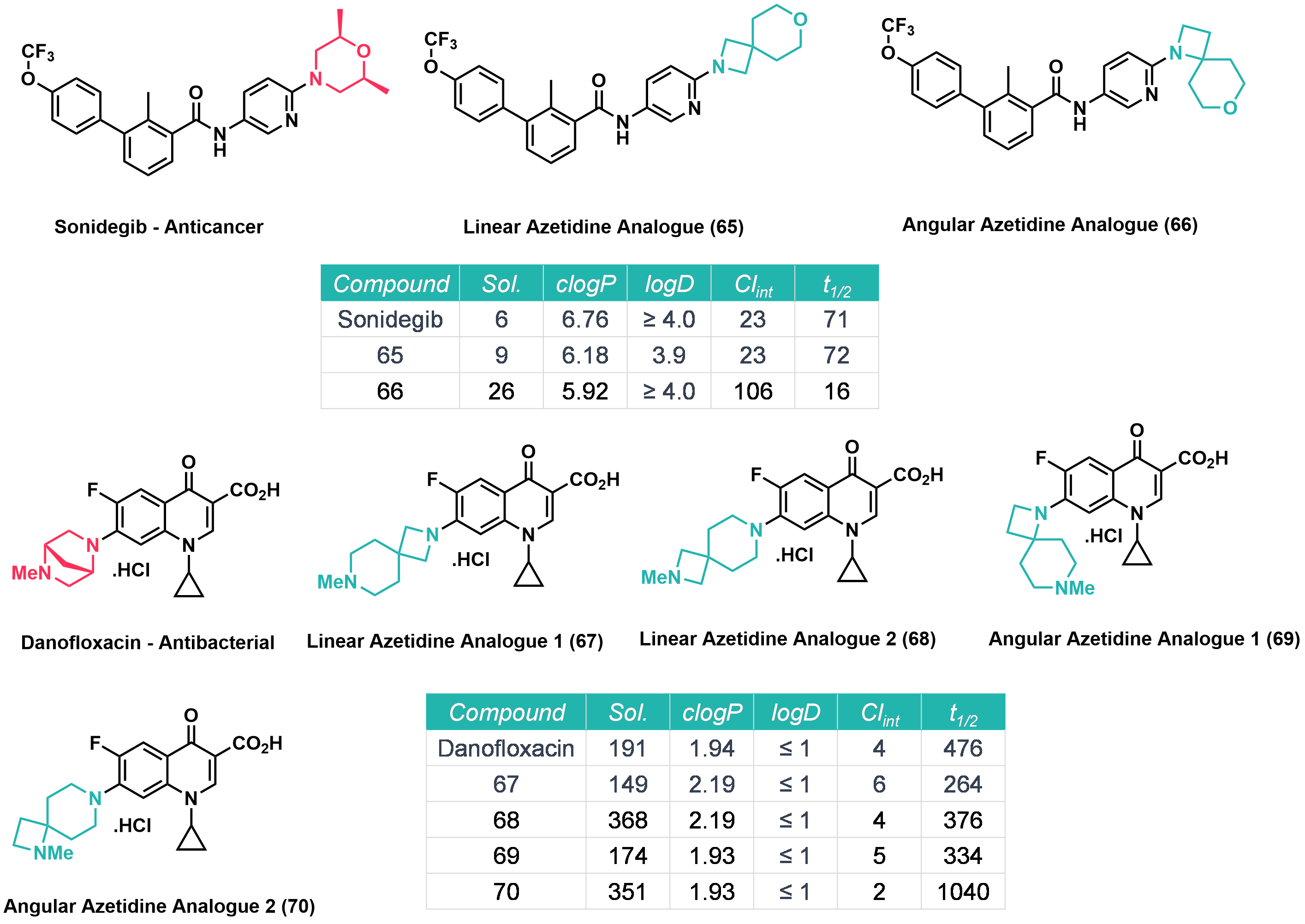
Figure 1: Azetidine analogues and biological data.
Light-promoted aromatic denitrative chlorination
Nitroarenes are a widely available class of feedstock chemicals. However, due to the (hetero)aromatic C-NO2 bond being relatively inert, methods for denitrative functionalisation via direct substitution remain elusive. SNAr can be a useful approach, however due to the high activation barrier requirement, it is typically limited to electron deficient nitroarenes. Therefore, a multistep functional group interconversion strategy is often employed whereby the nitroarene undergoes reduction to the corresponding aniline, followed by diazotisation to the aryldiazonium salt and finally Sandmeyer reaction to afford an aryl halide whereby further functionalisation is more accessible. Whilst the steps involved are typically robust, the 3-step strategy can suffer from poor atom economy, excess waste and requires the formation of diazonium salts, which are considered explosion risks (Scheme 2).
Whilst direct denitrative functionalisation has been explored in recent years via Pd-catalysis,3 opportunity for improvement and advancement in the field remained. To this end, the authors2 have developed an operationally simple procedure for denitrative chlorination of nitroarenes via visible-light (violet) irradiation. Furthermore, the conditions employed for the protocol are mild and no photoredox catalyst is required (Scheme 2).
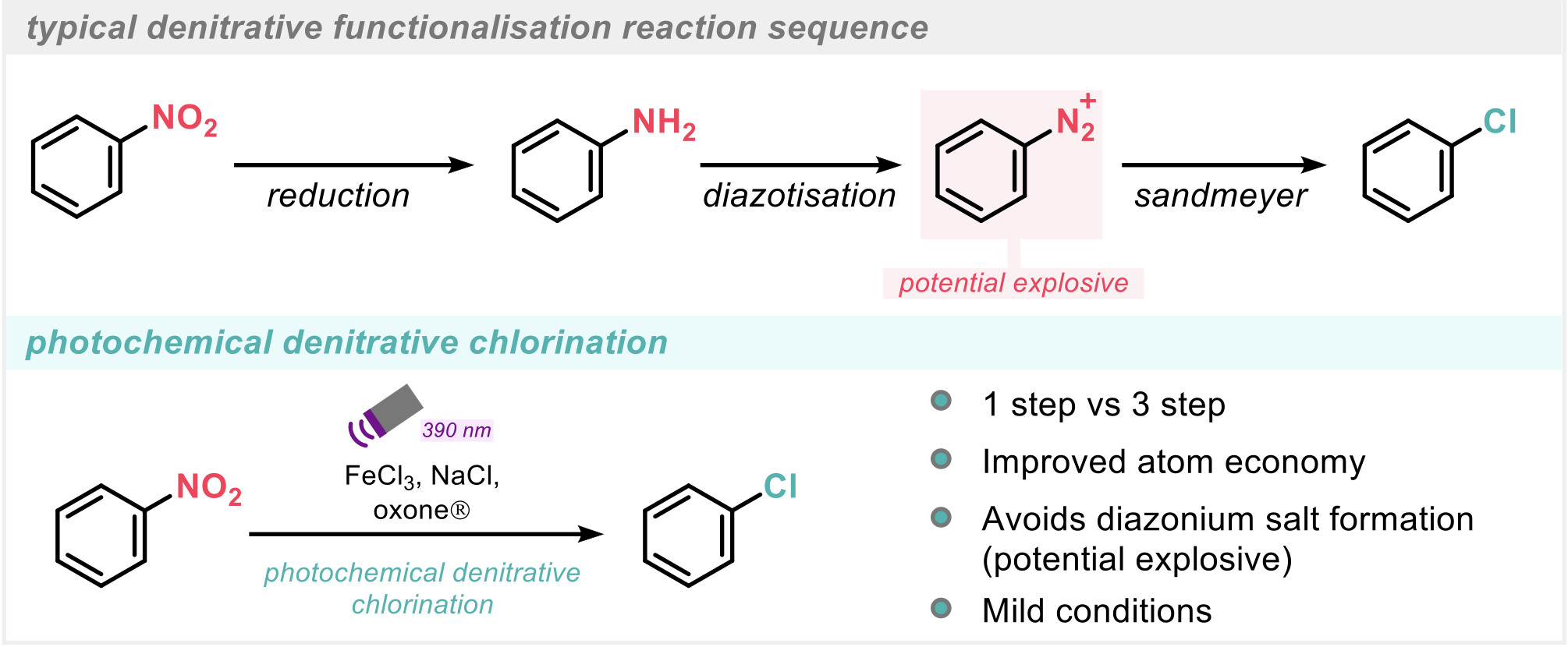
Scheme 2: Typical denitrative functionalisation reaction sequence.
The protocol makes use of 390 nm (violet) light to induce a ligand-to-metal charge transfer (LMCT) of first row transition metal chlorides to generate chlorine radicals. After initial studies, FeCl3 was identified as the best metal catalyst. Further optimisation and control studies demonstrated that the inclusion of Oxone® and NaCl improved the yield of the reaction, likely due to an increased concentration of chlorine radicals. Interestingly, it was shown that FeCl3 is not necessary for the reaction, however the efficiency is greatly reduced in its absence. The reaction is also able to proceed under an ambient air atmosphere, highlighting the robustness of the method.
A wide range of nitroarenes were shown to undergo the photochemical denitrative chlorination effectively including a selection of heterocyclic nitroarenes. Notably, substrates bearing electron donation groups such as free hydroxyl and phenolic ethers performed well in the reaction. These substrates were also tested for reactivity using thermal SNAr conditions, whereby they failed to afford product, highlighting the advantage of this photocatalytic method.
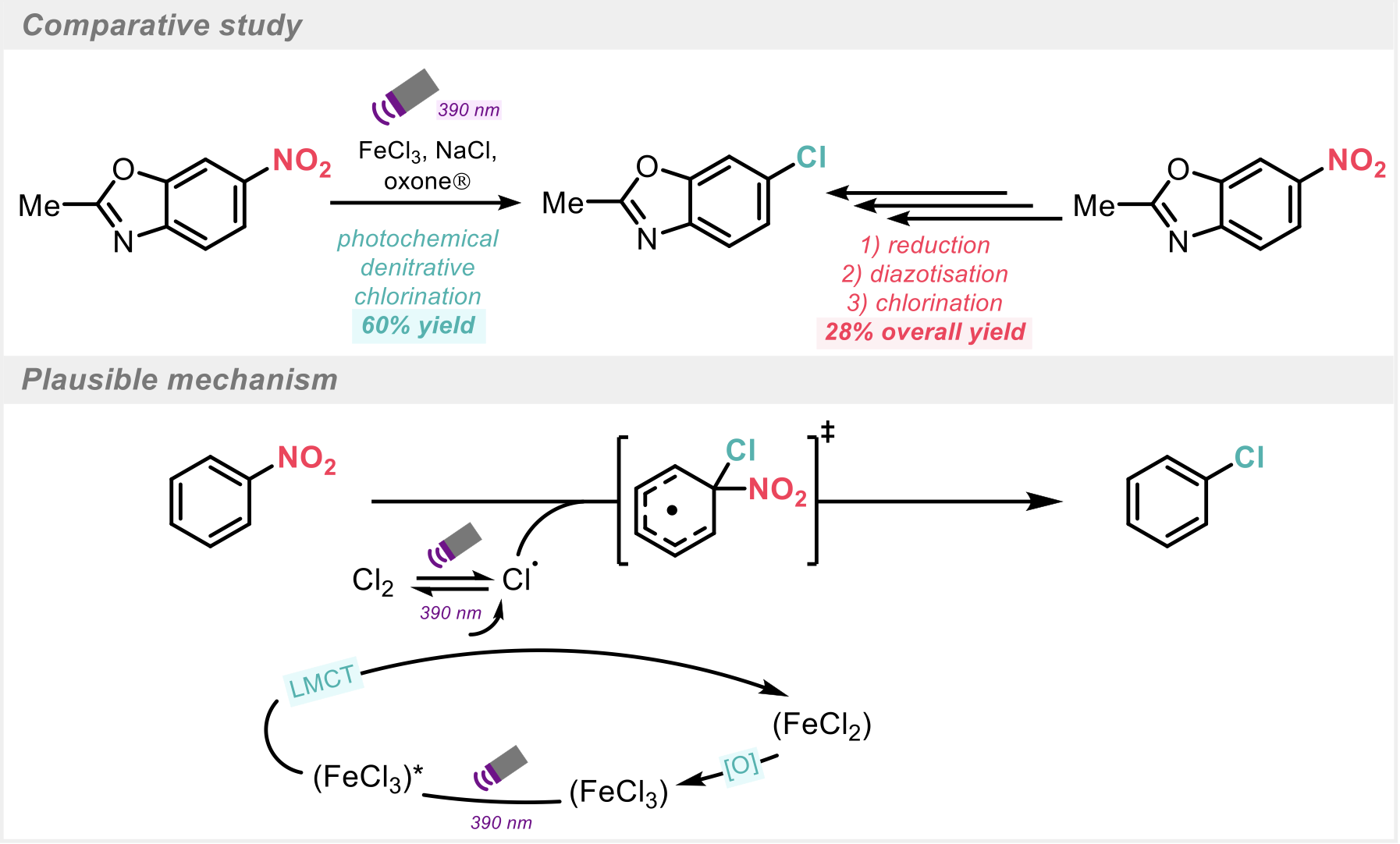
Scheme 3: Comparative study and proposed mechanism.
During a comparative study, the photochemical method outperformed C-H chlorination of both an electron-deficient nitroarene and an electron-rich nitroarene. The process was also directly compared to the commonly used 3 step reduction sequence (reduction, diazotisation, and Sandmeyer chlorination), (Scheme 3). In this case, the direct photochemical denitrative chlorination proceeds in a 60% yield and the 3-step sequence affords an inferior 28% overall yield.
Mechanistic studies gave rise to a plausible mechanism (Scheme 3) whereby dissociation of chlorine radicals occurs as a result of LMCT from the chloride ligand on the ferric ion. The generated free radical can then undergo radical substitution on the nitroarene to generate the chlorinated product and NO2 gas.
If you have a unique synthetic or medicinal chemistry challenge, our experts at Domainex are eager to collaborate with you. The angular spirocyclic azetidines work will be further explored within the realm of medicinal chemistry in our ongoing projects. Additionally, our expertise in photochemistry and the availability of specialised equipment will enable us to implement the light-promoted aromatic denitrative chlorination work in-house.
Get in touch to speak to one of our experts.
Additional Reference:
3. M Kashihara and Y Nakao, Acc. Chem. Res. 2021, 54, 2928−2935