By the Domainex Synthesis Group (Alicia Galván Álvarez, Claire Sear, Hugh Tawell, John May and Tenin Traore)
In the latest edition of our blog series, we have focused on the following two recent publications which described highly useful synthetic transformations:
- Deaminative chlorination of aminoheterocycles. Clément Ghiazza, Teresa Faber, Alejandro Gómez-Palomino and Josep Cornella. Nature Chemistry, 2022, vol 14, 78-74.
- Multicomponent double Mannich alkylamination involving C(sp2)–H and benzylic C(sp3)–H bonds. Zhencheng Lai, Rongkai Wu, Jiaming Li, Xing Chen, Linwei Zeng , Xi Wang, Jingjing Guo, Zujin Zhao, Hironao Sajiki & Sunliang Cui. Nature Communications, 2022, vol 13, Article number: 435
Accelerating the drug discovery process with deaminative chlorination of aminoheterocycles
Given the prevalence of heterocycles in biologically useful compounds, a simple method for converting the amino group of an aminoheterocycle into a leaving group would be a useful method of incorporating heterocycles in synthetic routes. The Sandmeyer reaction, a classic method of achieving this transformation, uses strongly oxidizing and acidic conditions, incompatible with many functionalities. In the recent publication by Josep Cornella et al1 at the Max Planck Institute, the authors draw inspiration from the Vilsmeier reaction, and develop a method to convert NH2 to Cl in heteroaromatic species using Cl- as a nucleophile in a mild and operationally simple transformation.
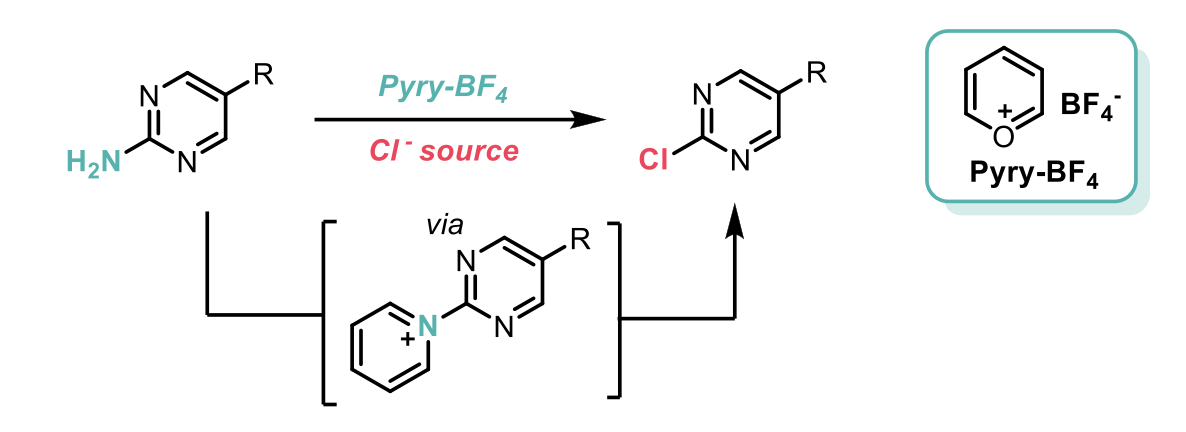
The mechanism of this process goes via the pyridinium tetrafluoroborate salt, through reaction of the amino group with the Pyry-BF4, followed by anion exchange with a chloride source. The reaction proceeds smoothly with a number of chloride sources, including HCl, TMSCl, MgCl2, and nBu4NCl, in a single flask set up with no special precautions required. The scope of this transformation is exceptionally broad and includes the late-stage functionalisation of an impressive array of commercial drug analogues and pesticides. A telescoped procedure was also developed, whereby an SNAr reaction was carried out on the chlorinated product, without the need for isolating the intermediate.
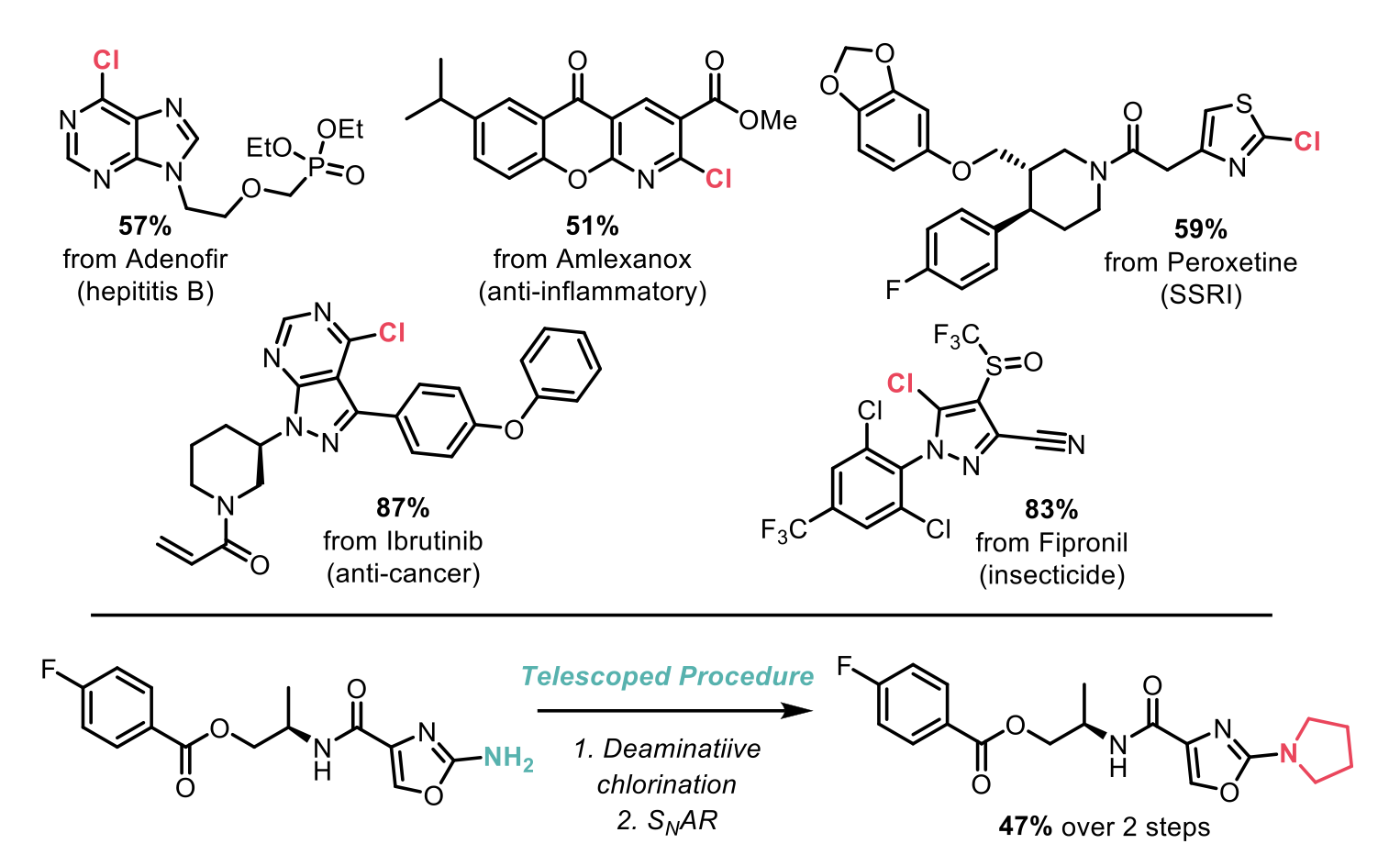
The simplicity and scope of this deaminative chlorination overall offers a simple tool for late-stage functionalisation and drug discovery.
Multi-component Mannich reaction for cyclic alkylamine formation
The 3-component Mannich reaction has been used extensively in synthesis, drug discovery and agro-chemistry for the formation of cyclic alkylamines, although the use of the reaction for unactivated C(sp3)-H bonds has remained a challenge. This research by Sunliang Cui et al2 overcomes this challenge by making use of benzylic C(sp3)-H bonds attached to electron-rich benzofurans.
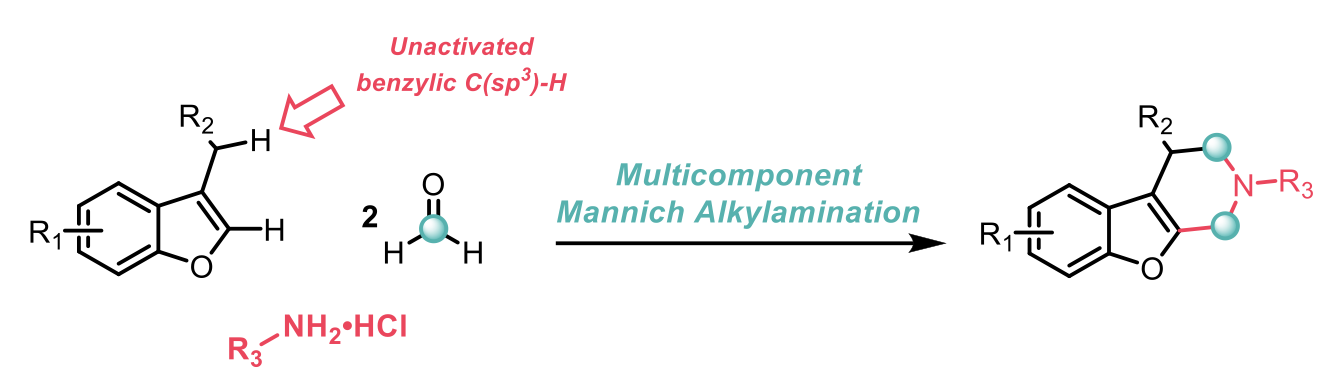
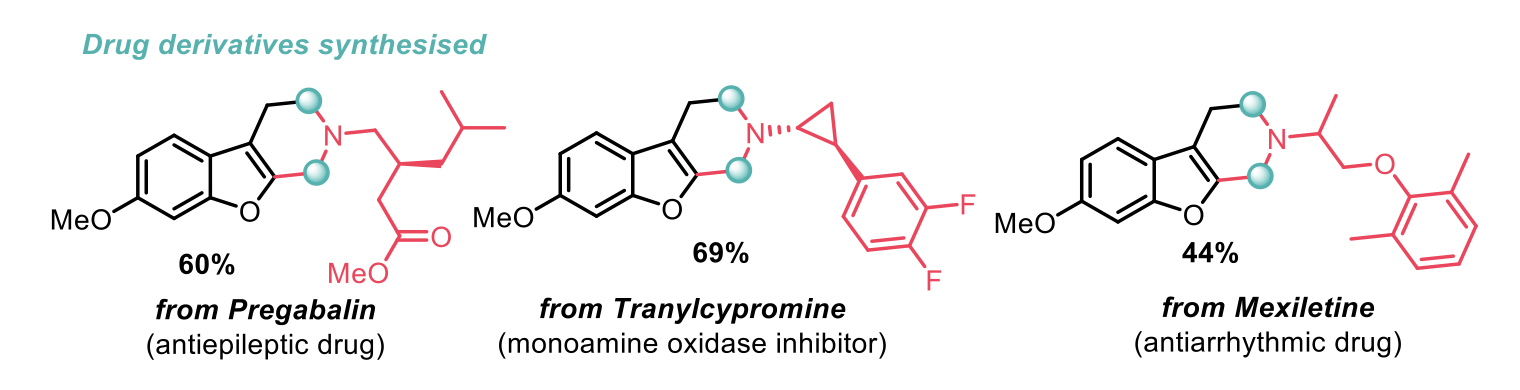
The conditions use either acetic acid or acetonitrile as the solvent, followed by treatment with base. The researchers demonstrated an impressive scope for the amine feedstock, including a number of amino acids, and sensitive functional groups such as alkynes, alkyl halides, esters and cyclopropyls. The late-stage functionalisation of some drug derivatives was also achieved and the corresponding products are shown below. The group were also able to modify the benzylic C(sp3)-H source to include useful synthetic handles, such as alkoxy-, bromo- and boronate.
A thorough mechanistic investigation was carried out by the group, including control experiments, kinetic isotope effect (KIE), and radical trap experiments, as well as a density functional theory (DFT) calculation to confirm the mechanistic hypothesis. The authors propose the reaction proceeds via multiple Mannich reactions and a retro-Mannich reaction, and dehydrogenation of the benzylic C(sp3)-H bonds to give the cyclized amine products.
Our synthesis team are always keeping an eye on the latest research to make sure we are employing the best approaches for our drug discovery projects. The next review will be available soon, but in the meantime, get in touch to find out how we can help solve your drug discovery challenges.