By Nick C Martin
The FDA recently granted fast track designation to the Pfizer developed Ervogastat/Clesacostat combination treatment for Non-Alcoholic Steatohepatitis (NASH). This has caught the eye of research staff at Domainex, predominantly because of the unorthodox tissue targeting mechanism of the Acetyl-CoA Carboxylase (ACC) inhibitor; Clesacostat.
Work published by Pfizer in 20201 details an intriguing campaign, involving development from pan-ACC inhibitor 3, the progress of which was hindered by extrahepatic ACC-mediated reductions in platelet count. To mitigate this, the team introduced characteristics that would make the series a substrate for Organic Anion Transporting Polypeptides (OATPs), key mediators of active hepatic uptake. Compounds recognised as OATP substrates are generally avoided, as higher hepatic uptake typically leads to faster metabolism and removal of the agent from systemic circulation. However, in this case, as the therapeutic target population of ACC is present in the liver, the strategy becomes more viable. It is reported that high MW anions increase OATP substrate affinity; therefore, the benzimidazole ring was exchanged and a carboxylic acid was added, affording a ~100 Da increase in MW and anionic character at physiological pH. The resultant compound Clesacostat (12) was found to be an OATP substrate, determined by comparison of hepatic clearance data in the presence and absence of the OATP inhibitor Rifamycin.
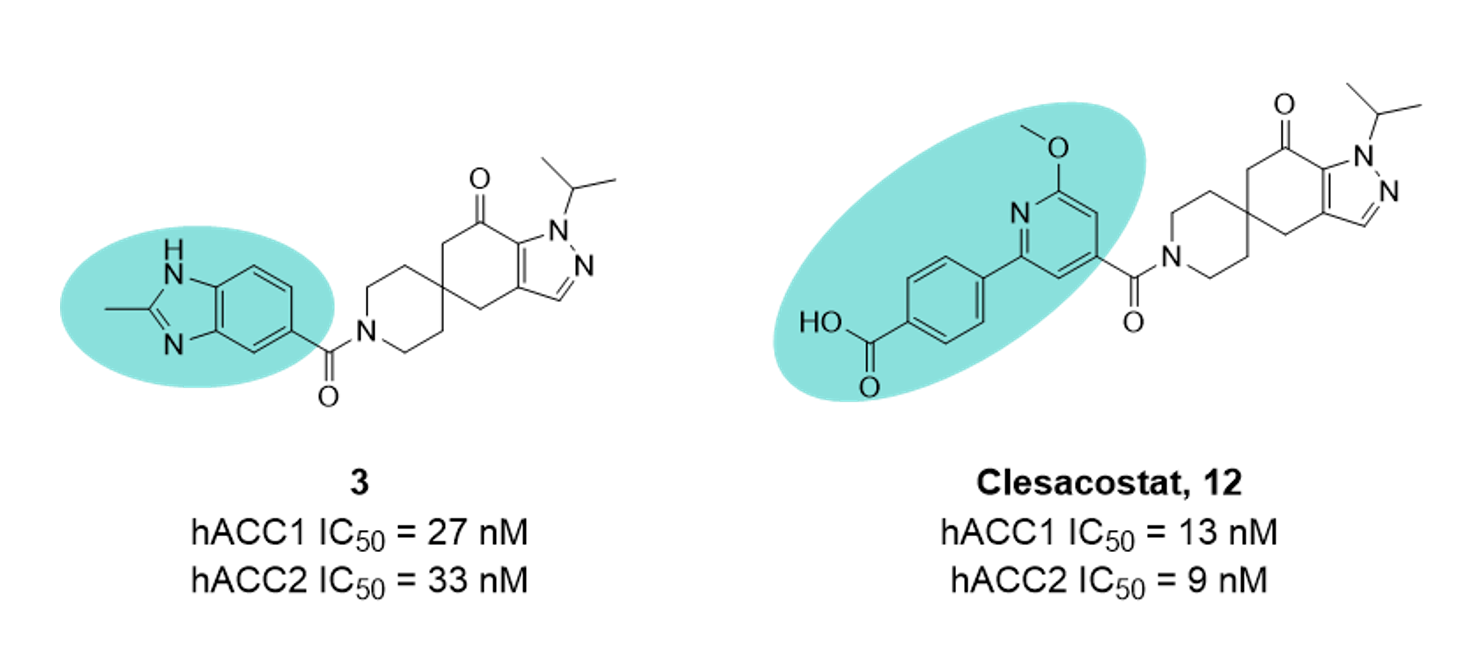
This strategy was only successful because Clesacostat possesses a very robust metabolic profile and therefore, despite being rapidly sequestered to the liver, still retains a good metabolic profile (t1/2 540 mins; human hepatocytes). We will wait eagerly to see the next round of clinical data!
1 Huard K, Smith AC, Cappon G, Dow RL, Edmonds DJ, El-Kattan A, Esler WP, Fernando DP, Griffith DA, Kalgutkar AS, Ross TT, Bagley SW, Beebe D, Bi YA, Cabral S, Crowley C, Doran SD, Dowling MS, Liras S, Mascitti V, Niosi M, Pfefferkorn JA, Polivkova J, Préville C, Price DA, Shavnya A, Shirai N, Smith AH, Southers JR, Tess DA, Thuma BA, Varma MV, Yang X. Optimizing the Benefit/Risk of Acetyl-CoA Carboxylase Inhibitors through Liver Targeting. J. Med. Chem., 2020, 63, 10879-10896