By the Domainex Synthesis Group (Andrea Bombana, Hugh Tawell, Andrew Jones, Venu Komanduri, Brahmam Medapi, Robyn Presland, and Vinny Duong)
In this blog post, we review two recent publications that highlight innovative and impactful synthetic methodologies in divergent photochemical strategies for skeletal editing; from C-to-N swaps to carbon deletion:
- C-to-N Atom Swapping and Skeletal Editing in Indoles and Benzofurans. Zhe Wang, Pengwei Xu, Shu-Min Guo, Constantin G. Daniliuc, and Armido Studer. Nature, 2025, 842, 92 – 98.
- Carbon-Atom Scavengers Enable Divergent, Selective Carbon Deletion of Azaarenes. Jisoo Woo, Tergitë Zeqiri, Alec H. Christian, Michael C. Ryan, and Mark D. Levin. J. Am. Chem. Soc., 2025, 147, 20120 – 20131.
Late-Stage Skeletal Editing of Indoles and Benzofurans via Photochemical Oxidation
Indoles and benzofurans are prevalent in numerous natural products and pharmaceutical compounds. In drug discovery programmes, it is sometimes necessary to perform skeletal editing of these heteroarenes to optimise potency or ADME (Absorption, Distribution, Metabolism, and Excretion) properties. Traditionally, this involves time-consuming, multistep classical heterocycle synthesis.
To address this challenge, the development of efficient synthetic methods for late-stage skeletal manipulation of heteroarenes is crucial. Studer et al1 present a novel and efficient oxidative skeletal editing strategy for indoles and benzofurans under mild photochemical irradiation conditions.
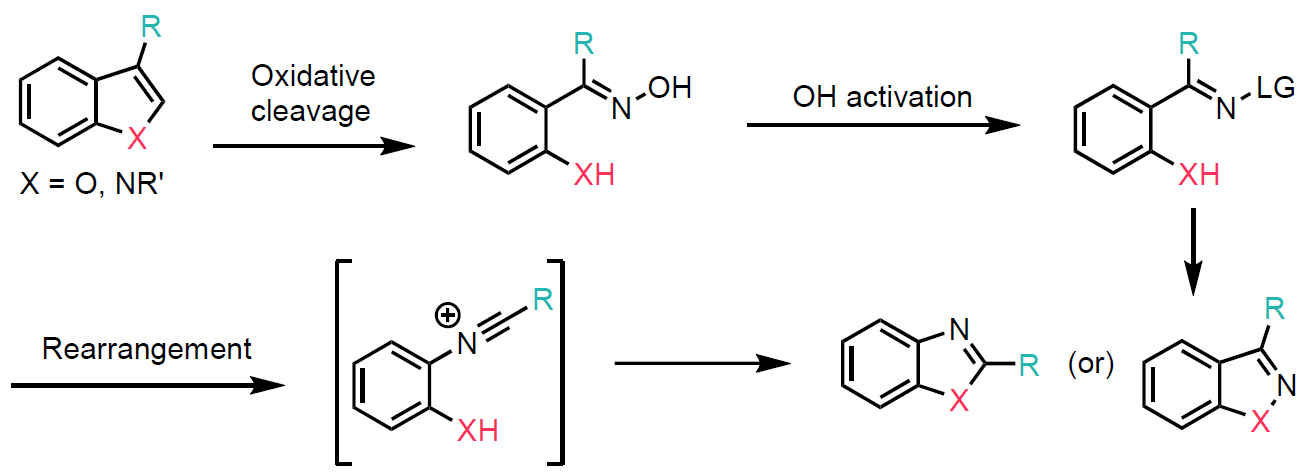
Scheme 1: Oxidative skeletal editing of indoles and benzofurans.
This transformation proceeds via a ring-opened oxime intermediate (Scheme 1). The authors began by investigating this oxidative ring-opening and skeletal editing approach using simple, commercially available indoles and benzofurans. The heterocycles were subjected to photoirradiation (3W, 415 nm) in ethyl acetate at room temperature, using N-nitrosomorpholine in combination with p-toluene sulfonic acid to generate the ring-opened oxime intermediate.
For indoles, this intermediate was further transformed under Mitsunobu or Beckmann reaction conditions to yield indazole or benzimidazole derivatives, respectively (Scheme 2, top left). Similarly, the hydroxyl group of the oxime intermediate was converted into a mesylate, affording benzisoxazole or benzoxazole derivatives (Scheme 2, top right).
This methodology demonstrates broad applicability across a variety of substituted indoles and benzofurans. Its potential is further showcased through successful transformations of complex natural products (Scheme 2, bottom left and right).
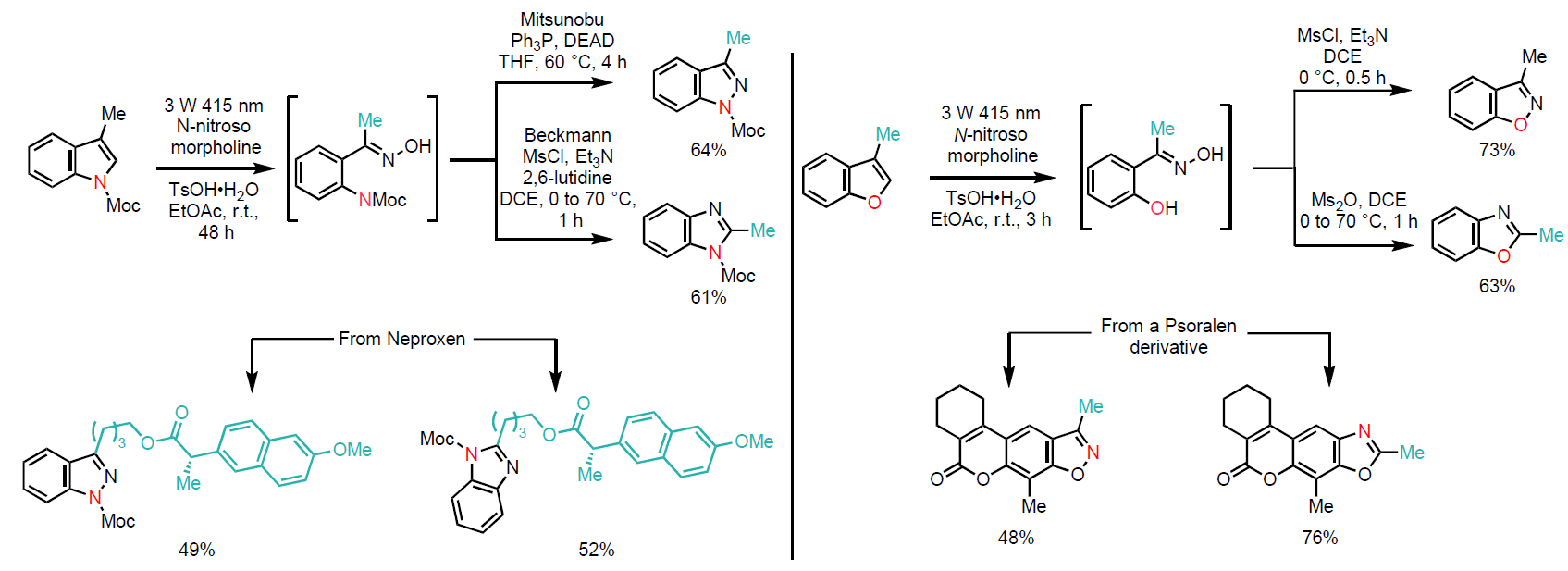
Scheme 2: Skeletal editing of indoles (top, left) and benzofurans (top, right) and application to natural products (bottom, left and right).
This innovative photochemical approach to oxidative skeletal editing of indoles and benzofurans represents a significant advancement in heterocyclic chemistry. By enabling late-stage diversification under mild conditions, this methodology offers a powerful tool for medicinal chemists seeking to fine-tune molecular scaffolds for improved biological and pharmacokinetic profiles. Its broad substrate scope, operational simplicity, and compatibility with complex natural products underscores its potential for widespread application in drug discovery and development.
Divergent Single-Atom Skeletal Editing of Quinolines: A Photochemical Approach to Indoles and Azaindoles
Single-atom skeletal editing has traditionally lacked divergent strategies for accessing multiple derivatives from a common substrate—an essential capability for exploring structure–activity relationships (SAR) in medicinal chemistry. Levin et al.2 introduce a reagent-controlled method using indoline or aminoethanol as carbon atom scavengers to selectively delete either the C3 or C2 carbon atom of quinolines and azaarenes (Scheme 3). This transformation is achieved via oxidation and 390 nm LED irradiation in a toluene/hexafluoroisopropanol (HFIP) solvent system, yielding indoles and azaindoles—scaffolds commonly found in FDA-approved drugs.
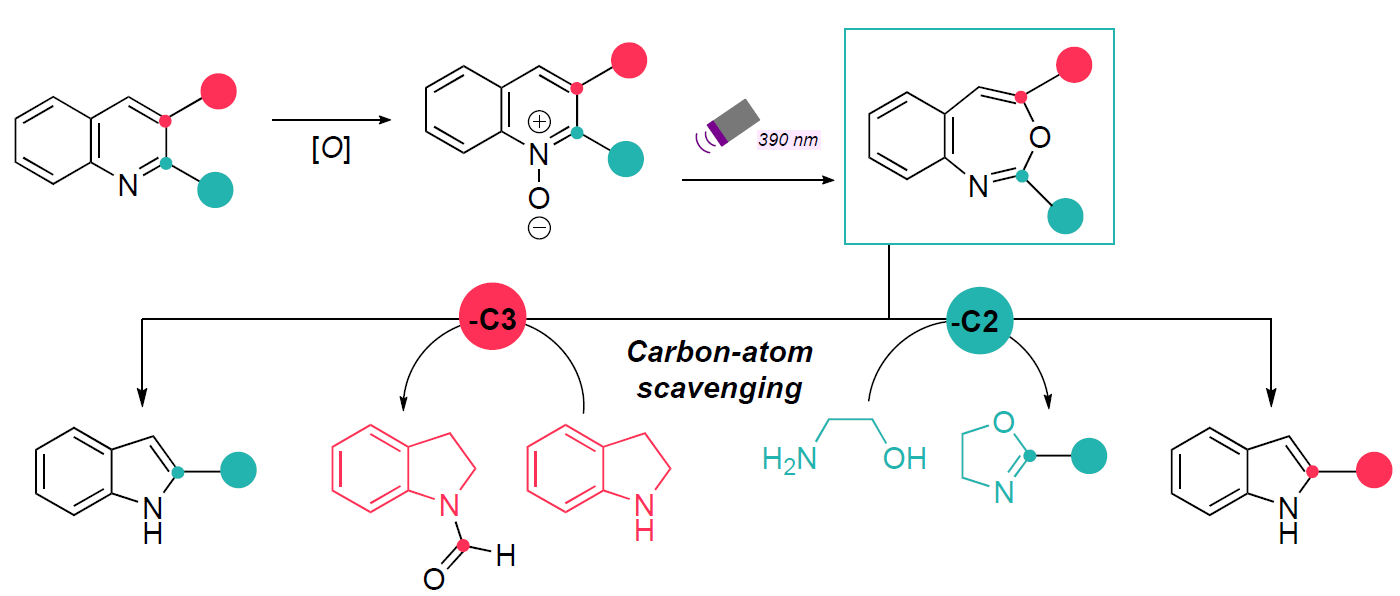
Scheme 3: Regiodivergent carbon deletion by carbon-atom scavengers.
Unlike previously reported skeletal modification strategies, where selectivity is governed by peripheral substituents3,4, this approach enables divergent synthesis from readily accessible quinoline precursors. Mechanistic studies reveal that C3-selective carbon deletion proceeds via photochemical generation of a 3,1-benzoxazepine intermediate, which subsequently reacts with two equivalents of indoline to form amidine I and bis(indoline) II (Scheme 4). This intermediate then cyclises to a zwitterionic species (III), which after elimination of the aminal indoline moiety yields the indoleiminium intermediate (IV). Two product-committing pathways were found in agreement with the Hammet data to explain the formation of the indole products. The first one involves the aminal (V) to rearomatize and generate the C3-deleted indole product (VI) after elimination of the formamidinium byproduct. The second plausible pathway describes the rearomatization of IV by deprotonation at the indoline C3 to generate VII, which results in formation of product VIII upon hydrolysis.
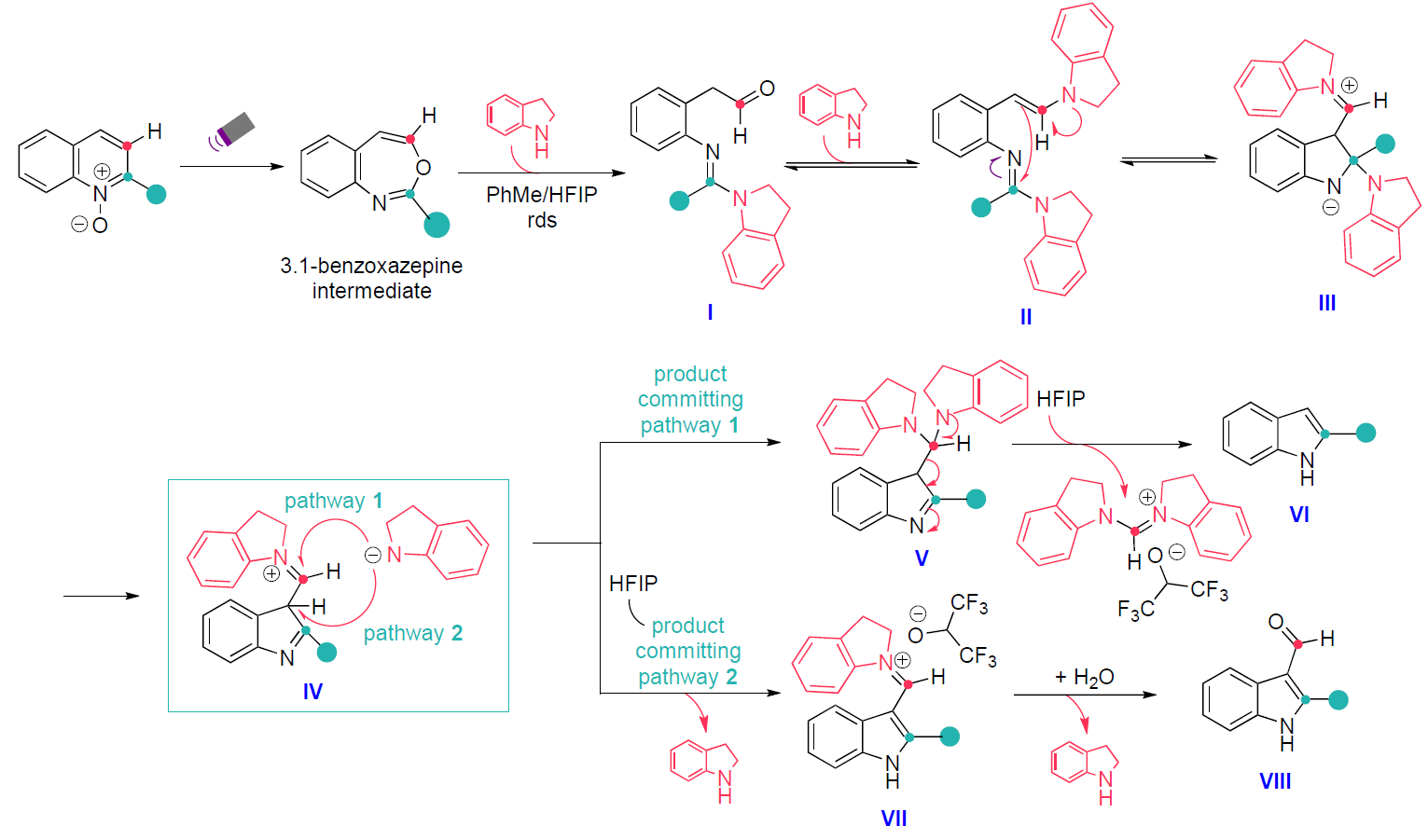
Scheme 4: Mechanism of C3-selective carbon atom deletion.
Similarly, C2-selective carbon deletion follows a comparable pathway (Scheme 5). The benzoxazepine intermediate reacts with aminoethanol to form amidine IX, which undergoes rapid intramolecular cyclisation of the terminal alcohol to an oxazolidine (X). Subsequent cyclisation (XI) and C–N bond cleavage result in selective C2 carbon deletion and formation of the indole product XII.
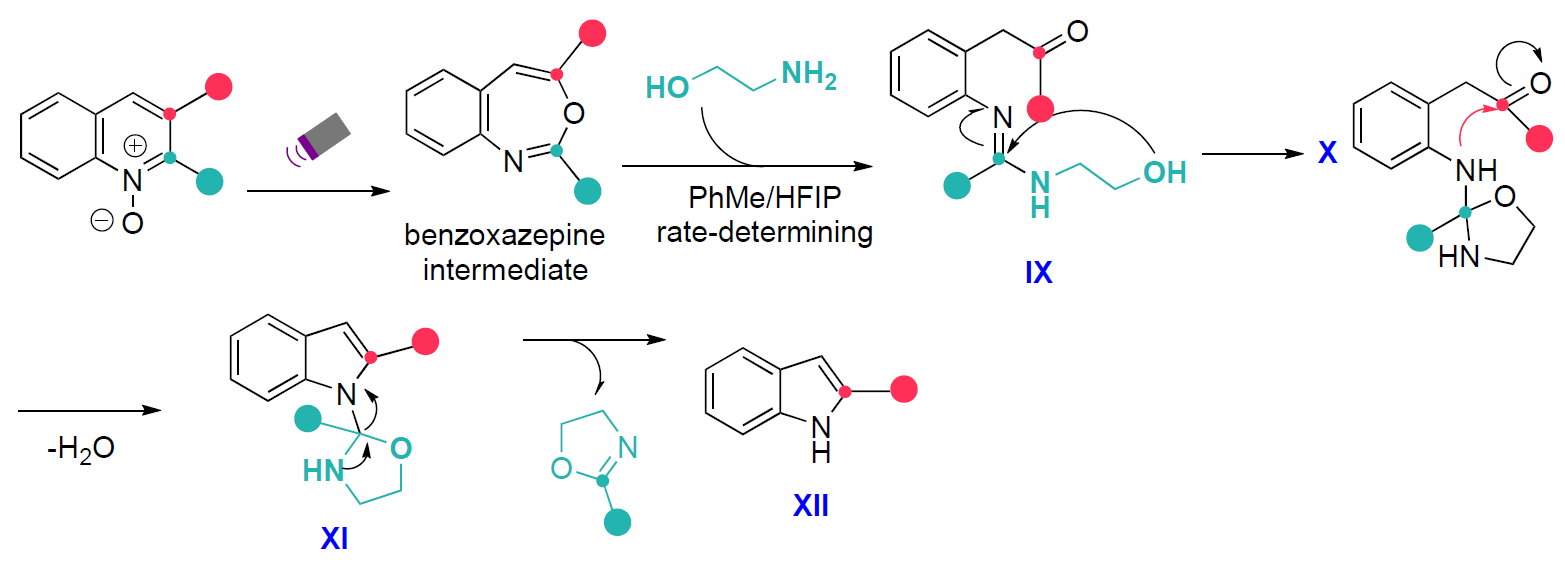
Scheme 5: Mechanism of C2-selective carbon atom deletion.
In summary, the authors demonstrate that indoline and aminoethanol enable selective C3 and C2 carbon atom deletion across a broad range of substituted quinolines. However, the strategy was found to be ineffective for substrates bearing heteroatom substituents at the 3-position (e.g., methoxy, bromo) and for 2,3-disubstituted naphthyridine analogues. Importantly, the study highlights how sequential edits—such as deletion followed by insertion—can facilitate unconventional peripheral modifications and enable late-stage skeletal editing, albeit with moderate yields.
Additional references
3. Fu-Peng Wu, Jasper L. Tyler, and Frank Glorius, Acc. Chem. Res. 2025, 58, 893-906.
4. Fu-Peng Wu, Jasper L. Tyler, Constantin G. Daniliuc, and Frank Glorius, ACS Catal. 2024, 14, 13343−13351.
Whether you are facing a complex synthetic chemistry challenge or seeking expert guidance to accelerate your medicinal chemistry programme, our scientists at Domainex are ready to partner with you every step of the way. With a passion for innovation and a track record of success, we are committed to driving discovery and delivering leads that make a real difference in your pipeline.