By the Domainex Synthesis Group (Alicia Galván Álvarez, Hugh Tawell, Tenin Traore, Andrew Jones, Jonás Calleja and David Gibson)
In the latest edition of our blog series, we have focused on the following publication:
Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane; Nils Frank, Jeremy Nugent, Bethany R. Shire, Helena D. Pickford, Patrick Rabe, Alistair J. Sterling, Tryfon Zarganes-Tzitzikas, Thomas Grimes, Amber L. Thompson, Russell C. Smith, Christopher J. Schofield, Paul E. Brennan, Fernanda Duarte & Edward A. Anderson, Nature, volume 611, pages 721–726 (2022).
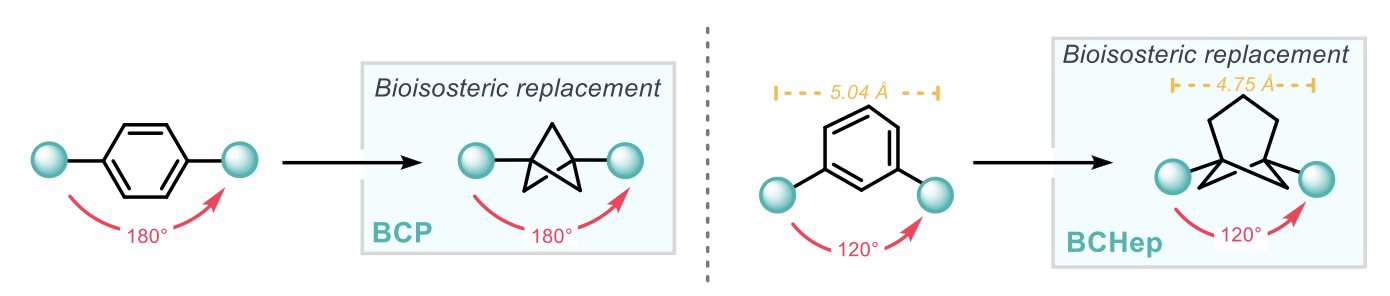
Small-ring cage hydrocarbons have an important use as bioisosteres for aromatic motifs due to their superior pharmacokinetic properties, for example one of the most commonly used strategies is the replacement of planar para-substituted benzenes with bicyclo [1.1.1] pentanes (BCPs). Whilst there have been numerous bioisosteres for para- and ortho- substituted benzenes, a geometrically accurate bioisosteric replacement for meta- substituted benzenes has, until now, presented a much greater challenge, with previous efforts not being able to fully reproduce the bond vectors of the substituents. This work by Anderson and co-workers presents a solution to this challenge through the utilisation of a bicyclo[3.1.1]heptane (BCHep) scaffold, whereby the bridgehead substituent vectors precisely mimic those of meta- substituted benzenes (120°).
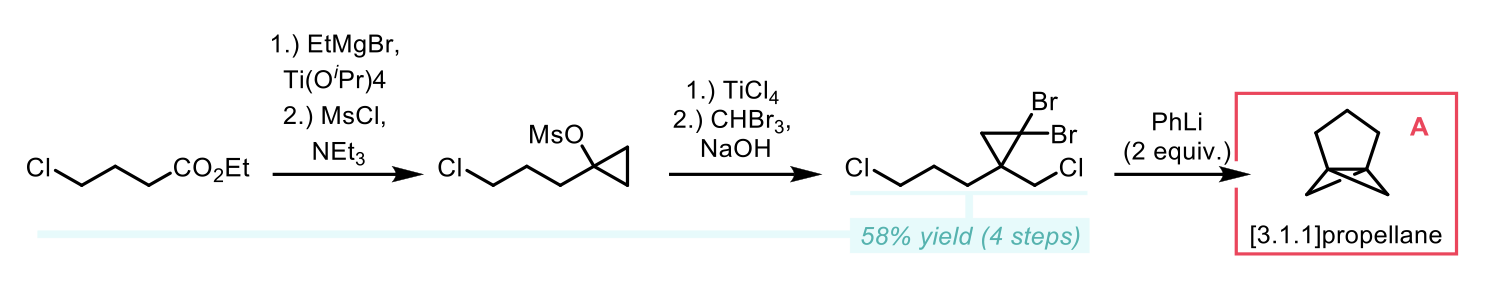
Previous methods for the synthesis of BCHeps rely on ring expansion of BCPs, or the cyclisation of cyclohexane dicarboxylates. These methods have limited applicability in terms of substrate scope and require lengthy and/or low yielding synthetic sequences. The authors here describe a direct, convenient synthesis of BCHeps from [3.1.1]propellane (A, a homologue of the precursor used for the synthesis of BCPs). Multigram scale preparation of this versatile intermediate is achieved through a short, efficient synthetic pathway. Firstly, Kulinkovich cyclopropanation of ethyl 4-chlorobutyrate, followed by mesylation of the resulting alcohol gives an intermediate primed for cyclopropyl-allyl chlorinative rearrangement. Dibromocyclopropanation resulted in the formation of the cyclopropane intermediate in 58% yield over the 4 steps with only one chromatographic purification required. Treatment of this intermediate with 2 equivalents of phenyllithium afforded the BCHep precursor (A) in 43-61% yield after distillation.
Prior literature reports regarding the reactivity of A were limited to solvolysis and addition of thiophenol. Computational modelling by the authors predicted that A would be susceptible to the radical functionalisation methods previously used to access substituted BCPs. To this end, a variety of alkyl iodides and bromides were subjected to a ring-opening atom-transfer radical addition reaction with A; either using a photoredox-catalysed method with Ir(ppy)3 co-catalyst, or by using BEt3 as a radical initiator. The photoredox-catalysed process proved to have greater generality and returned higher yields in most cases allowing for the synthesis of BCHeps from α-iodo carbonyls, benzyl iodides, α-amino acid derivatives and heteroaryl iodides. This methodology could also be used for late-stage cycloheptylation to produce BCHep derivatives of drugs such as corticosterone and indomethacin. In addition, bridgehead BCHep amines (attractive bioisosteres for meta-substituted anilines) could be synthesised by a three-component dual-photoredox/Cu catalysed coupling of A with N-heteroarenes and iodonium dicarboxylates. Thioethers and selenoethers were also accessible through direct reaction of A with the appropriate precursor.
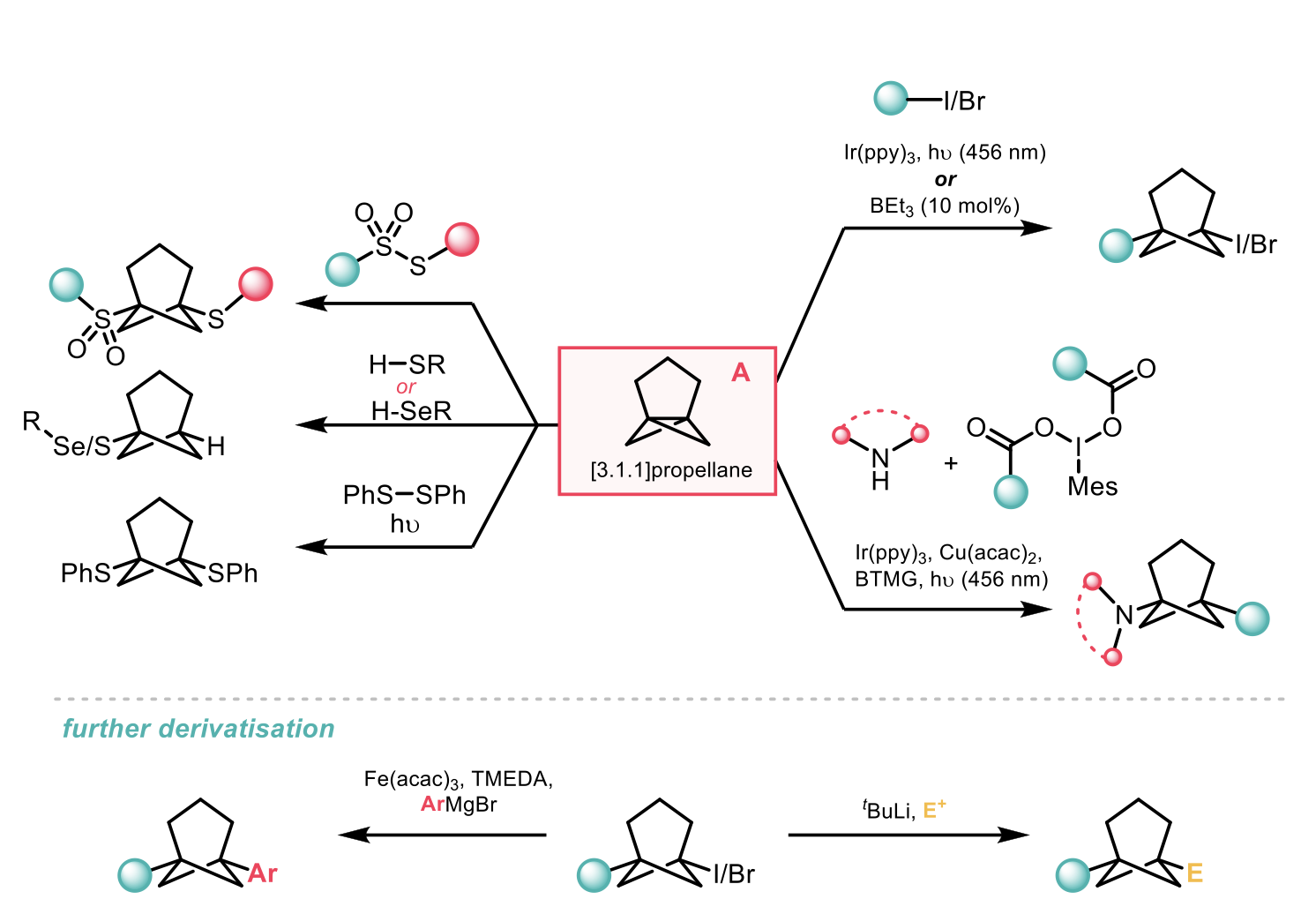
Further functionalisation of the iodinated BCHeps was possible through iron-catalysed Kumada coupling or via a lithiation/quench strategy.
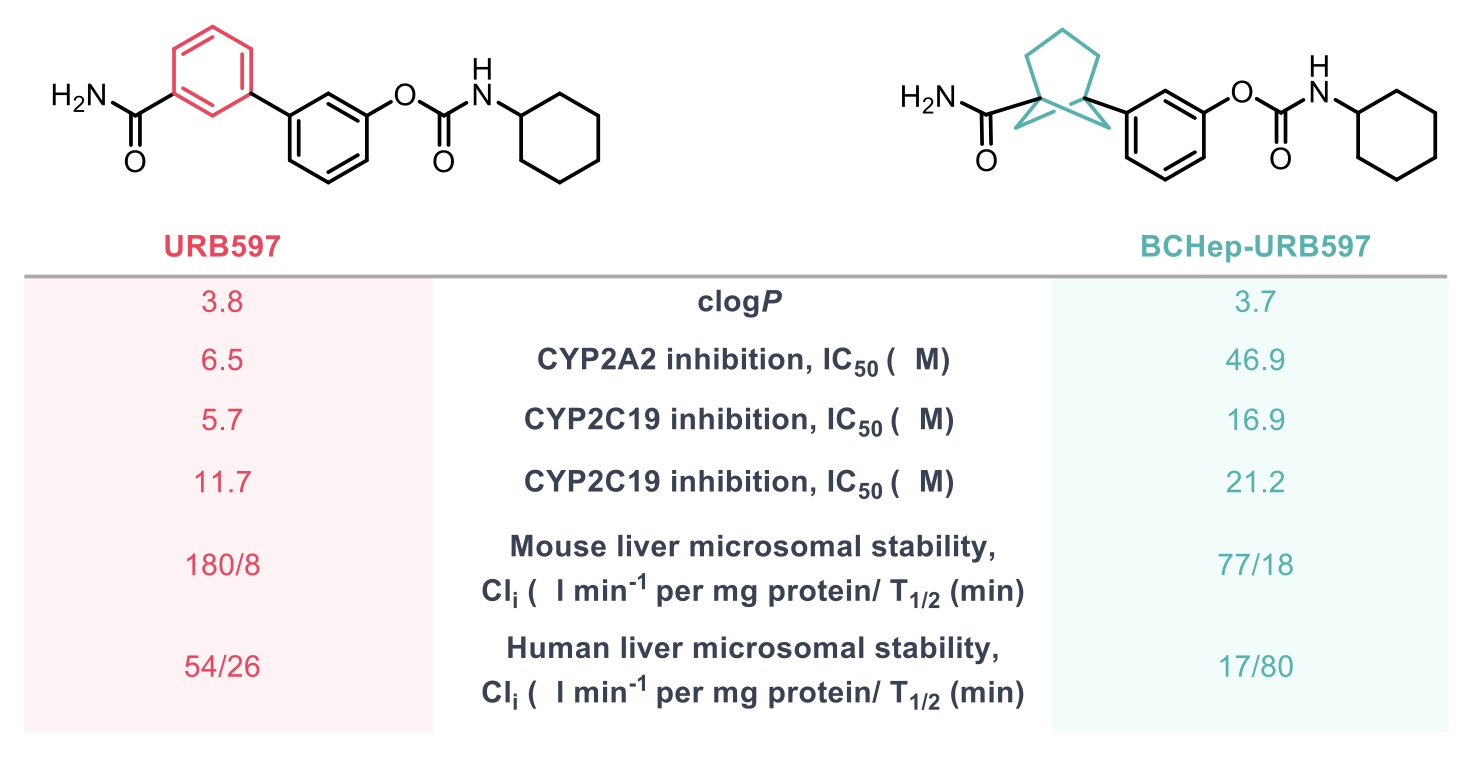
The BCHep bioisostere of the anti-cancer drug sonidegib was prepared to compare its physicochemical and pharmacological properties with those of the well-studied meta-phenyl drug. ClogP values were very similar for the drug and its BCHep analogue suggesting that the bioisostere would be a good replacement for the 1,3-disubstitued benzene present in sonidegib. Measurements on the BCHep compound showed an improvement in membrane permeability (Caco-2), consistent with its greater Fsp3. Similar results were found for anti-seizure drug URB597 and its BCHep analogue, and in this case, there was also an improvement in the microsomal stability and the CYP inhibition profile of the BCHep analogue versus the corresponding arene. The data from these studies represent a promising first investigation of the use of the BCHep scaffold to improve pharmacokinetic and pharmacological properties of drug candidates.