With the return to normal life since the beginning of 2022, Domainex is delighted to be able to resume its face-to-face meetings and welcome back external guest speakers to premises again.
Our ‘Science and Snacks’ events, inaugurated in 2019 but unfortunately put on hold in 2020 due to the COVID-19 pandemic, have now recommenced. The idea of these events is to invite guest speakers from all horizons to highlight and share the latest developments in scientific hot topics. Following the talks is an informal discussion in a relaxing atmosphere around a buffet of sweet and savoury snacks, as well as drinks.
At the early days of this event, we had the pleasure to welcome a number of prestigious speakers including Dr Rob Young (Blue Burgundy LTD, UK) talking about ‘Proteases in drug discovery’ and Dr Julian Blagg (Neophore, UK) presenting some insights into ‘Drug discovery past, present and future: a personal perspective’.
The development and applications of continuous flow processes
More recently, we have welcomed and really enjoyed Professor Marcus Baumann’s (University College Dublin, Ireland) visit and listening to his talk about ‘Continuous Flow Synthesis — Drug-like Scaffolds and New Cascade Products’. His research area is centered around the development of continuous flow processes and their application to synthetic challenges encountered in organic chemistry. His findings have enabled the generation and use of highly reactive intermediates, the synthesis of bioactive heterocyclic motifs, natural products and their analogues, and the use of modern continuous photochemical methods covering scales of up to kilograms.
Professor Baumann started his talk by highlighting the advantages of continuous flow processes, including improved heat and mass transfer, in addition to high spatiotemporal control due to reactor miniaturisation and high surface-area-to-volume ratio. He also discussed key applications, such as photochemistry (UV and Vis), heterogeneous reactions, exploiting high-energy intermediates, reaction telescoping and scale-up.
To demonstrate the medicinal chemistry applications, Professor Baumann and his team have been working on the synthesis of bioisosteres by investigating [1.1.1]propellane chemistry. The synthesis of this compound has been widely reported in the literature using batch chemistry, but not without several limitations, such as low reaction temperature, challenging small scale synthesis, distillation of the product required and the highly reactive nature of the product resulting in limited stability on storage. Professor Baumann described how, in order to overcome these issues, they designed and developed a continuous flow process which enabled them to generate [1.1.1]propellane solutions in only 6 minutes (-15°C to 25°C) with 50 per cent yield, independent of reaction scale.1
Bicyclo[1.1.1]pentanes (BCPs) represent valuable building blocks for medicinal chemistry purposes and are made via batch chemistry in multi-steps routes. Having a [1.1.1]propellane solution ‘on tap’ and mixing it with various chloro-oxoacetates using a new one-step conversion exploiting UV-light, Professor Baumann’s team has made possible access to mixed ester/acyl chlorides (Scheme 1) with 25-50 per cent yield in only 5 minutes, versus more than 24 hours previously published for both batch and flow chemistry.
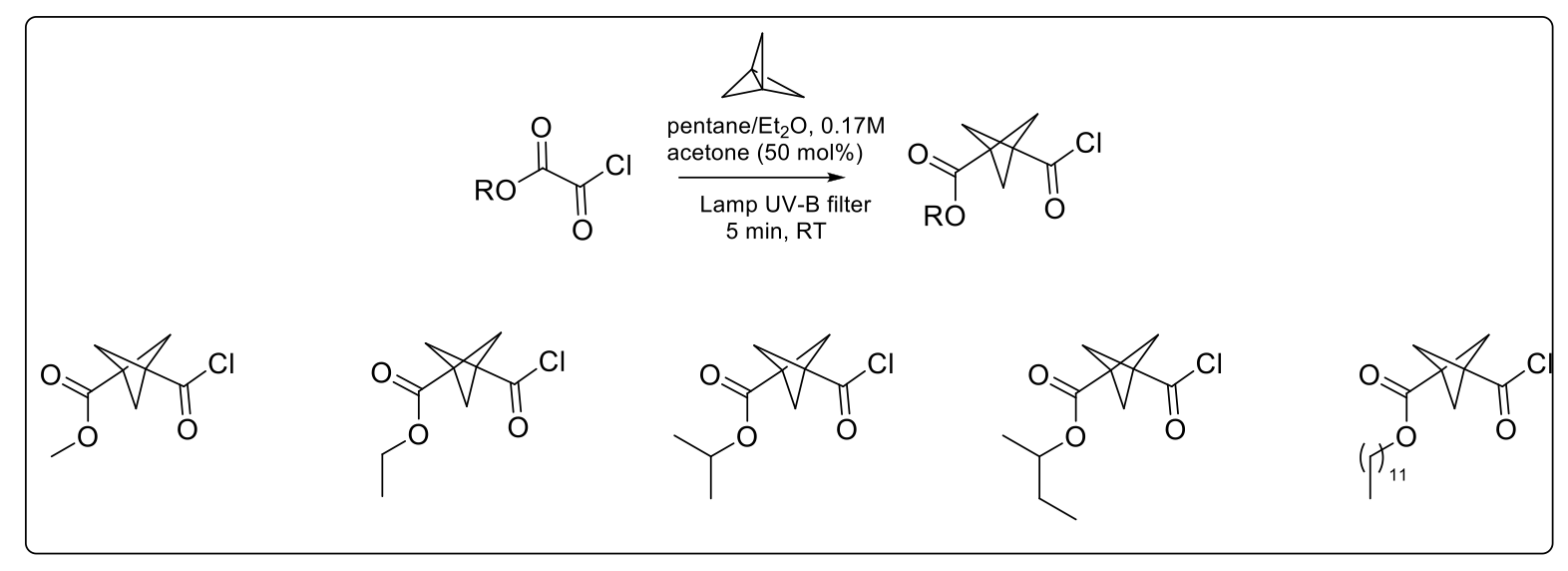
Scheme 1: Examples of mixed ester/acyl chlorides BCPs
After further work and exemplification, they obtained a library of extremely diverse and non-symmetrical acyl-BCPs; and consequently, rapid access to new BCP ester/amides and BCP-oxadiazoles2 (Scheme 2).
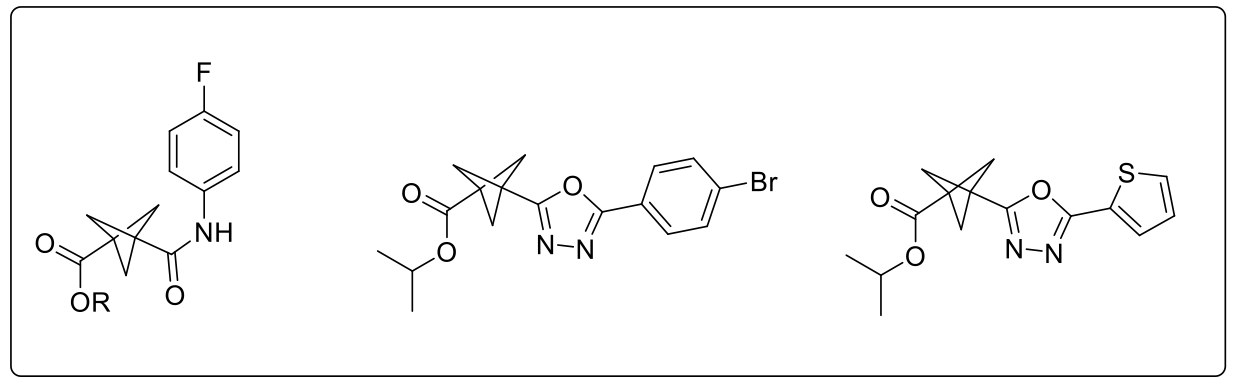
Scheme 2: Examples of BCP ester/amide and BCP oxadiazoles
Studies and synthetic efforts towards the synthesis of Cbz-carbamates using flow chemistry, although previously reported in the literature (for example by Naber et al3 and Marsini et al.4), have also been of interest to Professor Baumann’s group. Special attention was given to tandem processing and coupling of the Curtius rearrangement. There was also a strong desire to improve the safety of the reaction (use of toxic azide, exothermic reaction, etc.), but above all, developing an effective method for the challenging removal of the residual benzyl alcohol (bp= 205 °C) typically used in excess (2-5 eq.) was key. In order to achieve that aim, the group used an enzymatic impurity tagging strategy5 involving the use of an enzyme that derivatised the unwanted reagent, BnOH, into an easily removable by-product; all avoiding the use of chromatography. The process involved passing the reaction solution over immobilised Candida antarctica lipase B (CALB), up to 100 mmol over ~3.5 hours. Removal of the BnOH was monitored by 1H NMR and HPLC. Upon completion, the reaction mixture was evaporated and after extraction/evaporation, the pure product was obtained by simple trituration or crystallisation, resulting in a facile purification process under continuous flow.
Discovery of brand-new chemical space
Last but not least, the discovery of brand-new chemical space thanks to an unprecedented photocyclization reaction was an amazing and exciting part of Professor Baumann’s talk. Taking inspiration from quinoline-forming photo-processes, Professor Baumann’s group started studying the behaviour of analogous chalcones bearing an alkyne group in place of the amine, under the same conditions. The photochemical cascade process, a flow-based interception of isomerising alkenes, then demonstrated new chemical reactivity and produced complex chemical scaffolds6 by continuously generating and intercepting high-energy intermediates in a very efficient manner (supported by DFT calculations). The obtention of a crystal structure along with spectroscopic data were consistent with the presence of a cycloheptatriene as a key electrocyclisation intermediate and precursor of the structurally complex reaction product (Scheme 3).

Scheme 3: New photocascade showing unprecedented photocyclization discovered by flow chemistry
This general method afforded a variety of complex three-dimensional structures in good to excellent yields in a very short reaction time (7 minutes). Further investigations are currently ongoing in order to explore the scope and applicability of this very unique light-driven cascade process.
Domainex would like to thank Professor Baumann for his visit and fascinating talk.
Please get in touch if you would like to discuss how Domainex can apply synthetic chemistry approaches to solve your drug discovery challenges.
References
- Kian Donnelly and Marcus Baumann; Chem. Commun., 2021, 57, 2871-2874
- Kian Donnelly and Marcus Baumann; J. Org. Chem., 2022, 18, 232-239
- John R. Naber, C. Oliver Kappe and Jaan A. Pesti; Org. Process Res. Dev. 2020, 24, 2342
- Maurice A. Marsini, Frederic G. Buono, Jon C. Lorenz, Bing-Shiou Yang, Jonathan T. Reeves, Kanwar Sidhu, Max Sarvestani, Zhulin Tan, Yongda Zhang, Ning Li, Heewon Lee, Jason Brazzillo, Laurence J. Nummy, J. C. Chung, Irungu K. Luvaga, Bikshandarkoil A. Narayanan, Xudong Wei, Jinhua J. Song, Frank Roschangar, Nathan K. Yee and Chris H. Senanayake; Green Chem. 2017, 19, 1454
- Marcus Baumann, Alexander Leslie, Thomas S. Moody, Megan Smyth, and Scott Wharry; Org. Process Res. Dev. 2021, 25, 452-456
- Mara Di Filippo, Cristina Trujillo, Goar Sánchez-Sanz, Andrei S. Batsanov and Marcus Baumann; Chem. Sci., 2021, 12, 9895