- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Ras: PPI inhibitors for the treatment of cancer
Identification of inhibitors of RAS-effector protein interaction using a fragment-based drug design approach
Domainex employed a number of techniques and its extensive experience of fragment-based drug design to develop fragment hits which exhibited RAS binding. These fragment hits were developed into leads with increased binding affinity and improved physical properties.

Challenge
Activating RAS mutations are associated with approximately 30% of cancers; therefore, blocking RAS-effector interactions with biological reagents has beneficial effects in cancer models. Professor Terry Rabbitts of the University of Oxford, commissioned Domainex to help develop fragment hits into lead molecules, which bind potently to activated RAS and inhibit binding of effector proteins such as PI3K and Raf. This work was unprecedented when it was initiated.
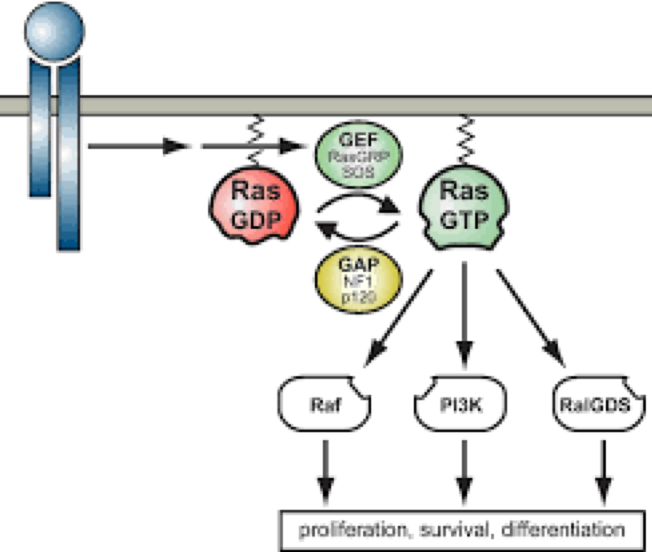
Solution
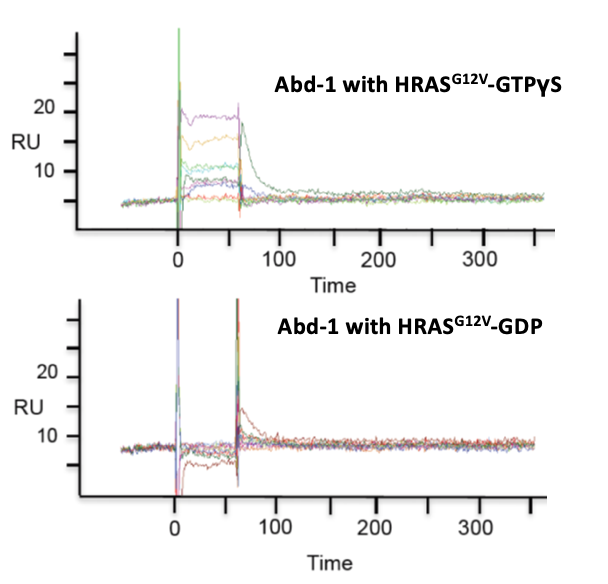
Prior to Domainex starting work on the programme, a fragment hit (Abd-1) that demonstrated selective binding to GTP-bound RAS (i.e. the active form) was identified from an SPR screen of a fragment library of 656 compounds.
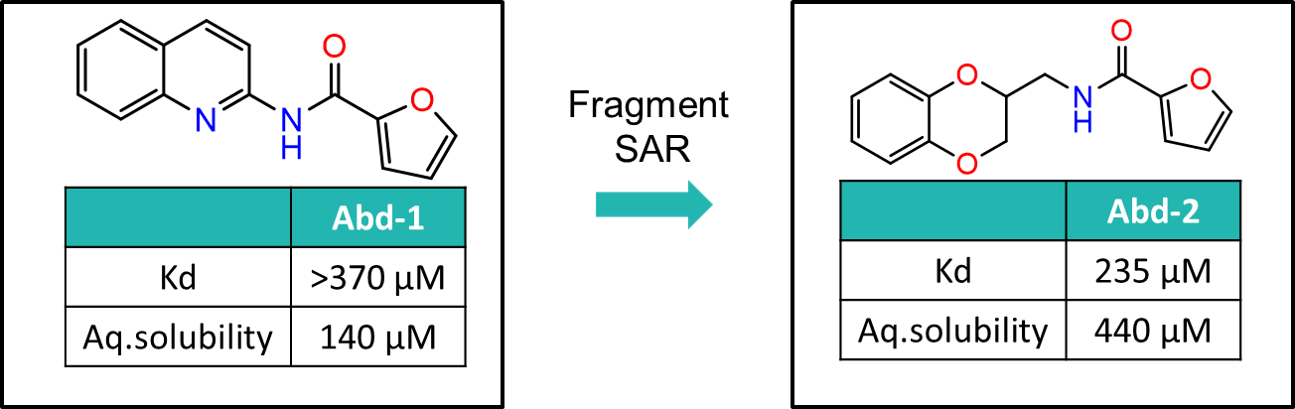
The SAR around Abd-1 was explored to generate Abd-2, which had increased RAS-binding affinity and improved aqueous solubility. These superior properties facilitated structural biology studies and enabled the binding mode to be confirmed by X-ray crystallography.
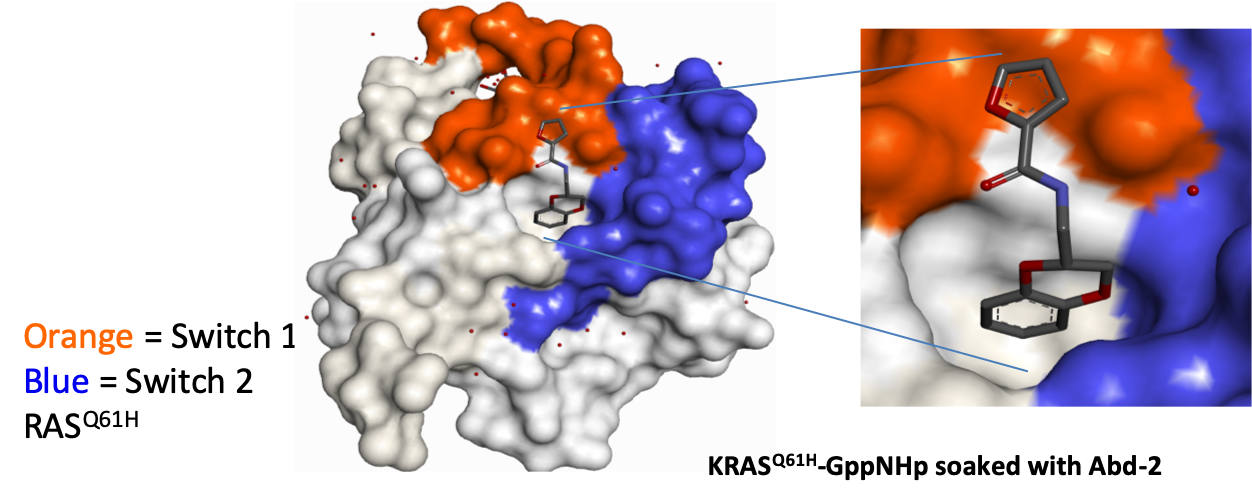
Domainex medicinal chemists then commenced work on the programme. We utilised our experience in fragment-based drug discovery and were able to develop the fragment hits into lead molecules using a structure-guided approach. RAS-binding was enhanced, and a solubilising substituent was introduced to enhance the physical properties in order to assist with the X-ray crystallography.
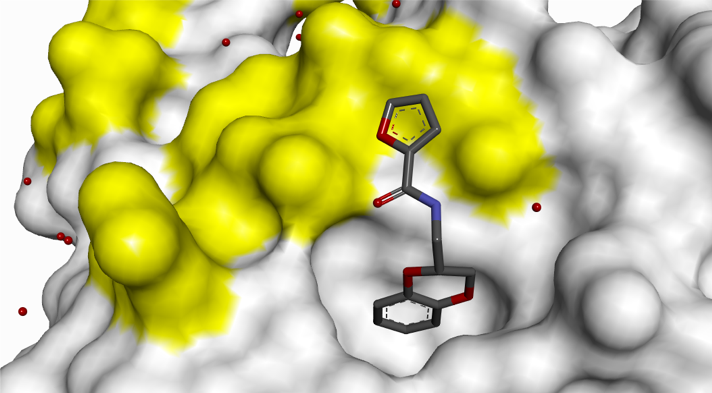
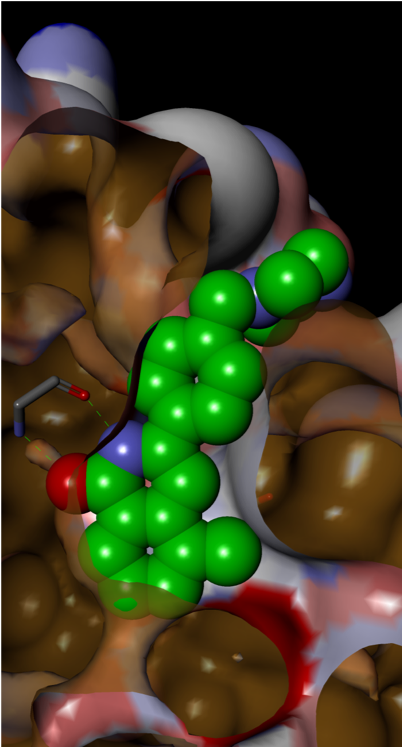
Small H-bonding groups proved to be ineffective in forming additional interactions with RAS, but an 8-pyridyl substituent showed a significant improvement in potency. X-ray crystallography showed that this induced a conformational change (see Figure 6):
- Asp-54 rotation opens up a lipophilic feature to accommodate aromatic group
- Arg-41 moves closer to pocket
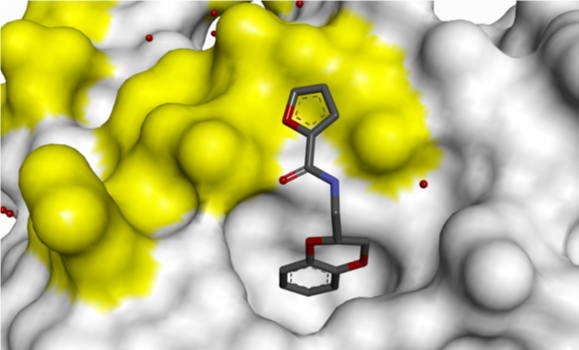
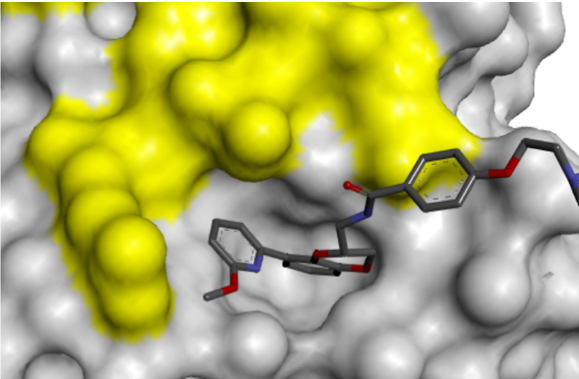
Figure 6: 8-Pyridyl substitution induced a conformational change
The conformational change presented an opportunity for growth towards the Switch 1 region, accessible via substitution on the pyridyl ring, which led to increased binding affinity.
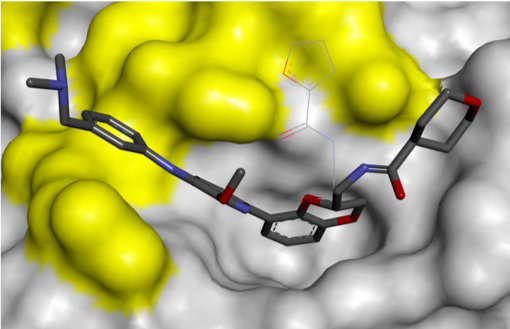
Finally, removal of the solubilising group increased ligand efficiency, resulting in the cell-permeable RAS PPI inhibitor, Abd-7. The Rabbitts group showed that Abd-7 disrupted the interaction of RAS with a range of effector proteins, and modulated signalling downstream of RAS, eliciting a reduction in phosphorylation of downstream proteins AKT and ERK.1
Conclusion
Domainex utilised its extensive experience of fragment- and structure-based drug design to elaborate a fragment hit into a lead molecule with efficient binding to RAS. This and related compounds were shown to inhibit the protein-protein interactions between RAS and effector proteins and tumour viability in cellular assays.
Domainex Expertise
• Medicinal and Synthetic Chemistry • Fragment-based Drug Design (FBDD) • Structure-based Drug Design (SBDD)
• DMPK and ADME • Lead Optimisation • Protein-protein Interaction Drug Discovery • Oncology
Reference
1. Small molecule inhibitors of RAS-effector protein interactions derived using an intracellular antibody fragment. Camilo E. Quevedo, Abimael Cruz-Migoni, Nicolas Bery, Ami Miller, Tomoyuki Tanaka, Donna Petch, Carole J.R. Bataille, Lydia Y.W. Lee, Phillip S. Fallon, Hanna Tulmin, Matthias T. Ehebauer, Narcis Fernandez-Fuentes, Angela J. Russell, Stephen B. Carr, Simon E.V. Phillips and Terence H. Rabbitts., Nat Commun 9, 3169 (2018).
Press release
A new class of RAS-effector protein-protein interaction inhibitors
20th December 2018
Start your next project with Domainex
Contact one of our experts today