- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- DMPK
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Spectral Shift Competition Assay: A robust and versatile assay to assess compound binding to a panel of kinases
Challenge
Competition assays using fluorescent probes are an excellent method for overcoming the difficulties of working with proteins that do not respond well to being fluorescently labelled or immobilised on a biosensor. These challenging targets can be characterised using fluorescence polarisation (FP)/anisotropy-based competition assays. FP assays are easy to set up and can produce very high-quality data. However, these assays usually require large amounts of protein, which makes them expensive and can cause problems with ligand depletion, limiting their ability to discriminate between potent compounds.
Solution
To overcome these issues, Domainex has developed a competition assay that utilises spectral shift technology. The use of an ATP-competitive fluorescent probe enables kinases to be studied in their native states and the sensitivity of the Dianthus instrument (NanoTemper) allows for far less protein consumption than a typical FP assay. The affinities of the probe were determined using both FP and spectral shift (Figure 1).
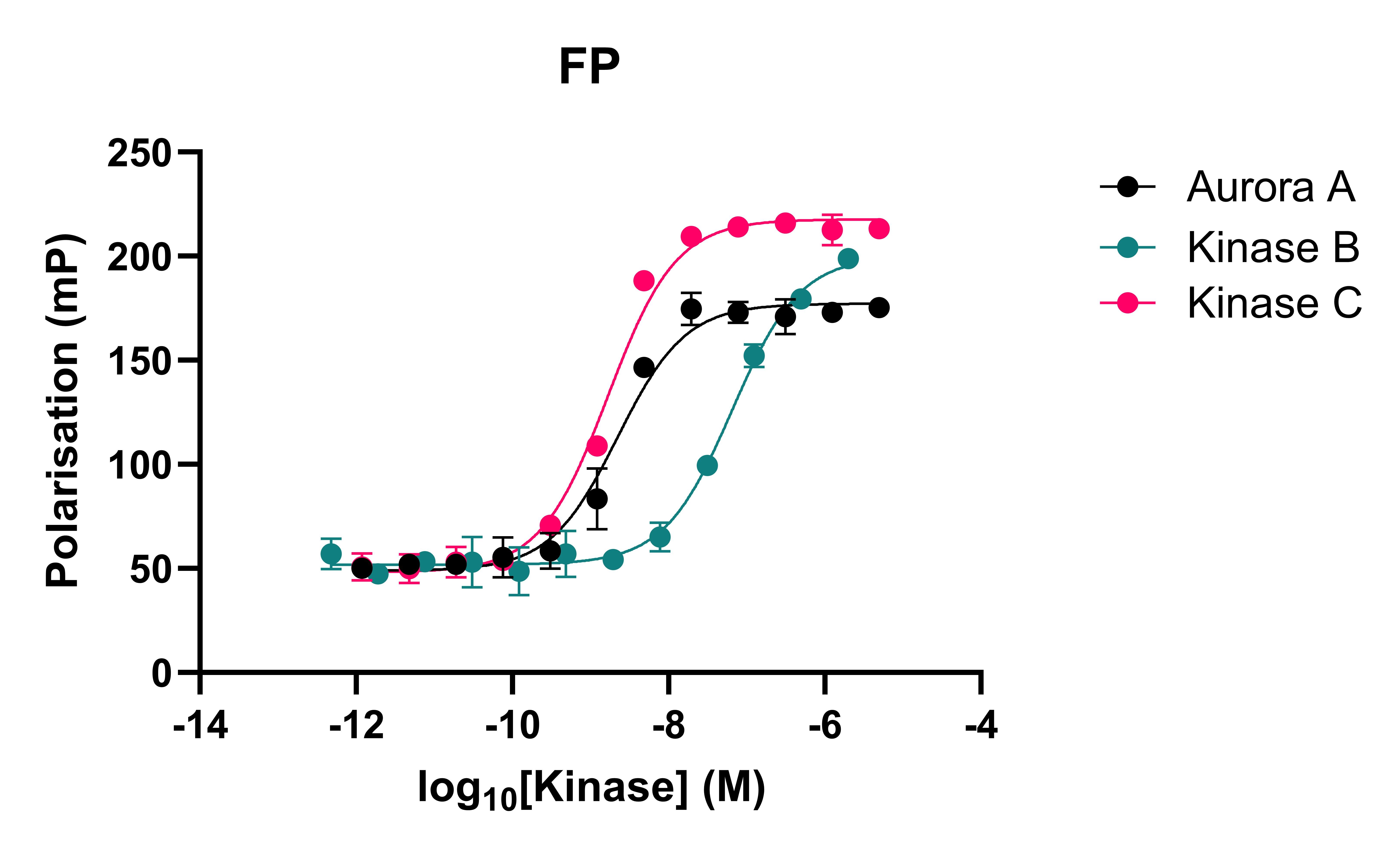
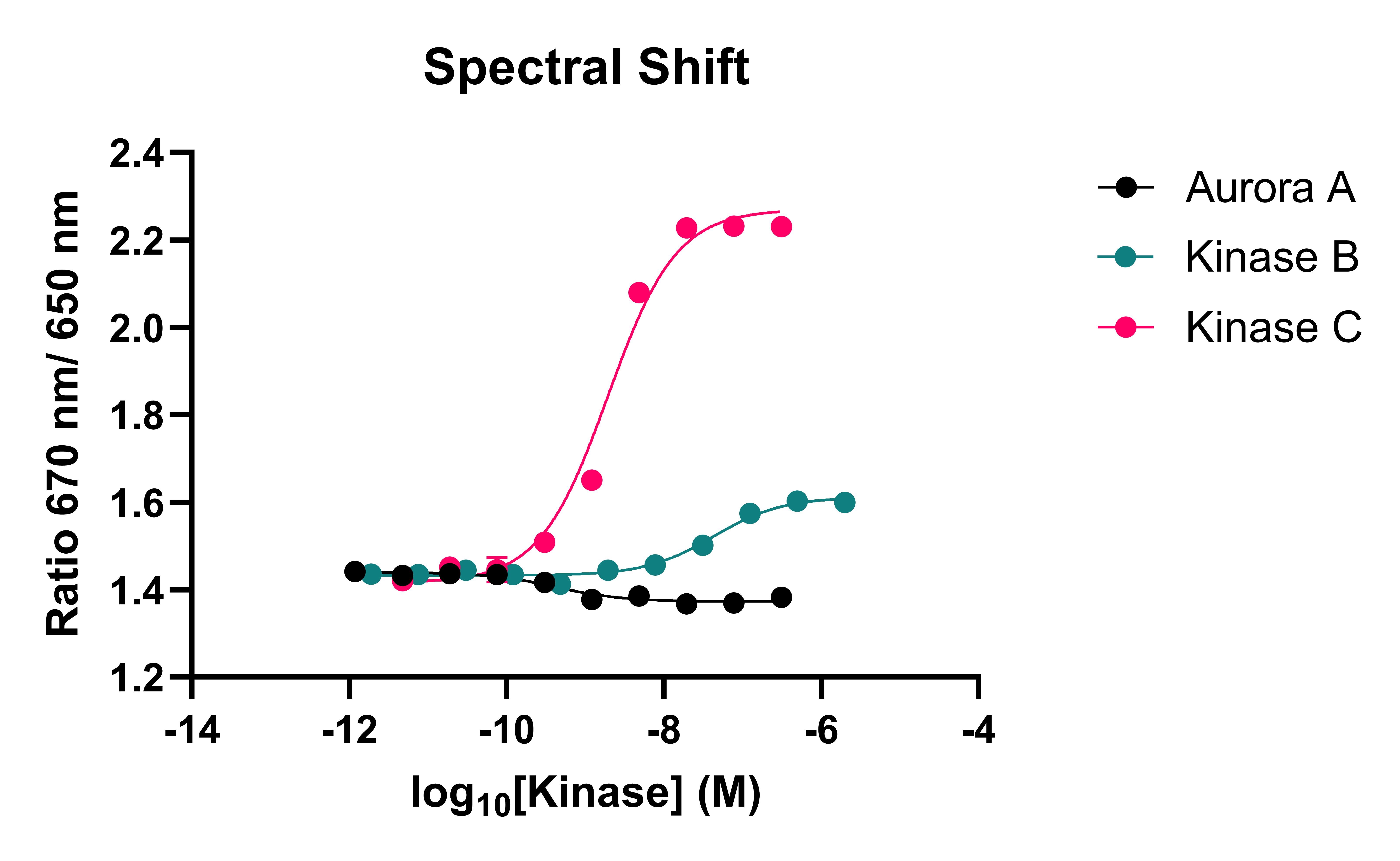
Figure 1: Kinases binding to the fluorescent probe in both spectral shift and FP assays
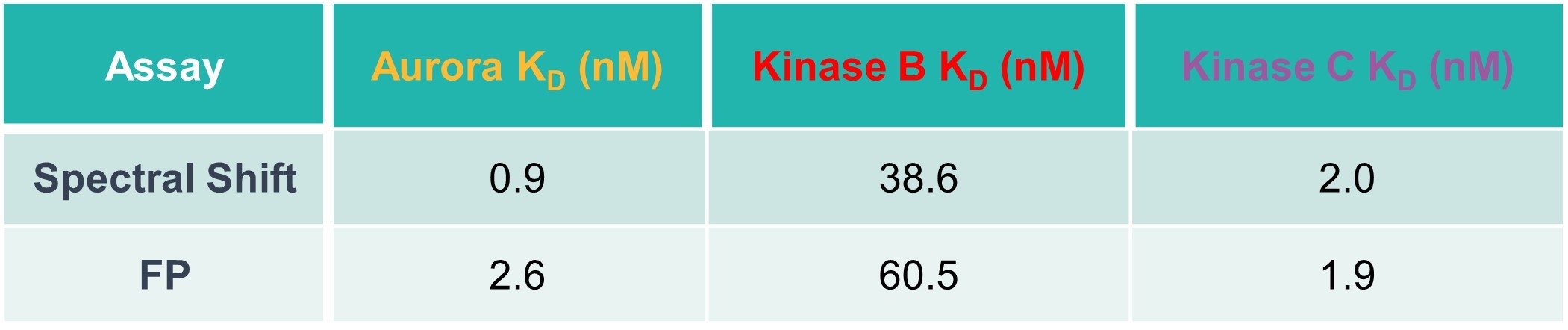
Table 1: Comparison of spectral shift and FP data. KD values for kinases binding to the fluorescent probe are provided.
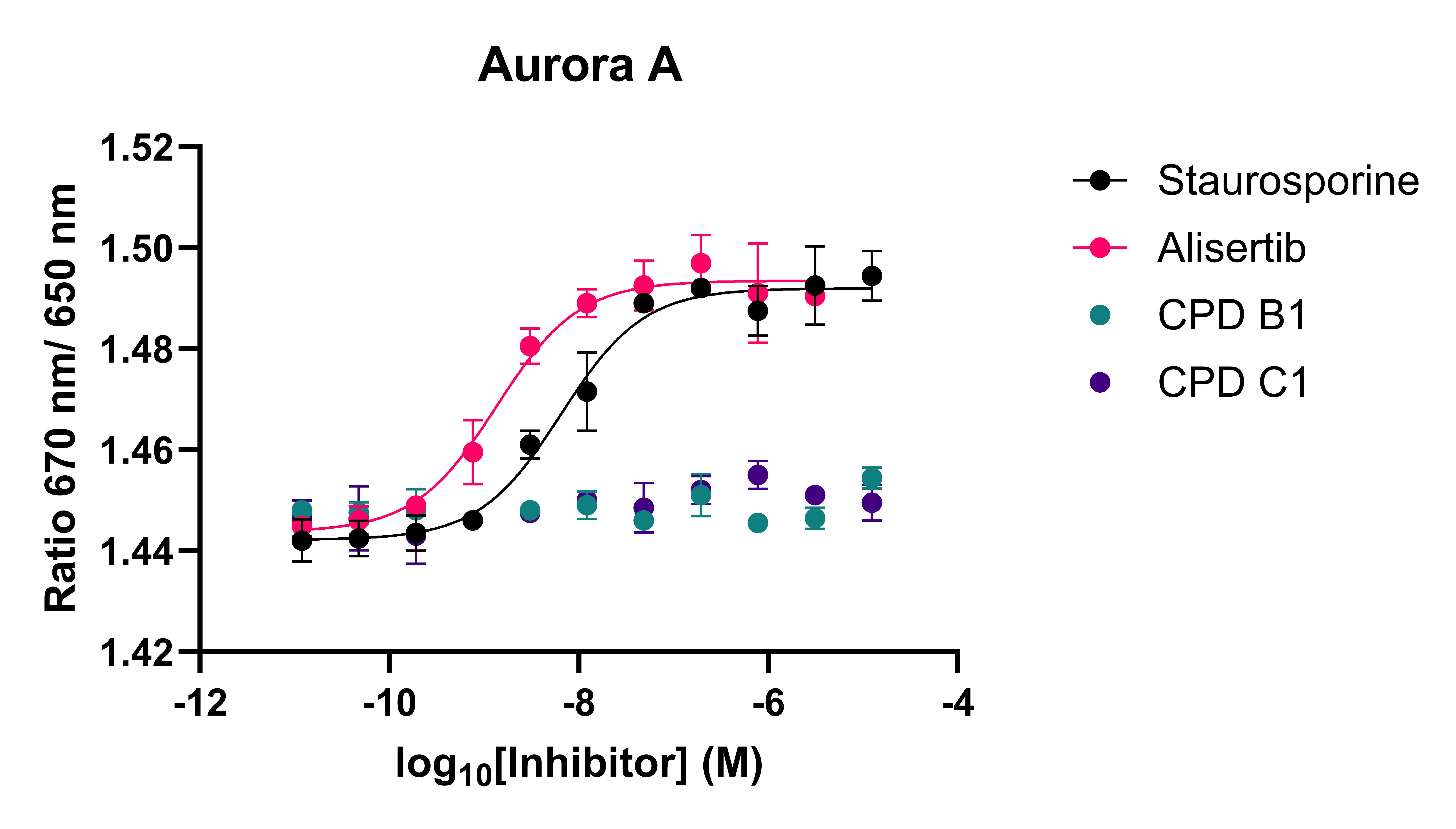
After assay validation and affinity determination, a panel of kinase inhibitors was tested against the protein-probe complex for disruption (Figure 2). The spectral shift assay had a good dynamic range with IC50 values measured from low nanomolar to high micromolar.
 .
. 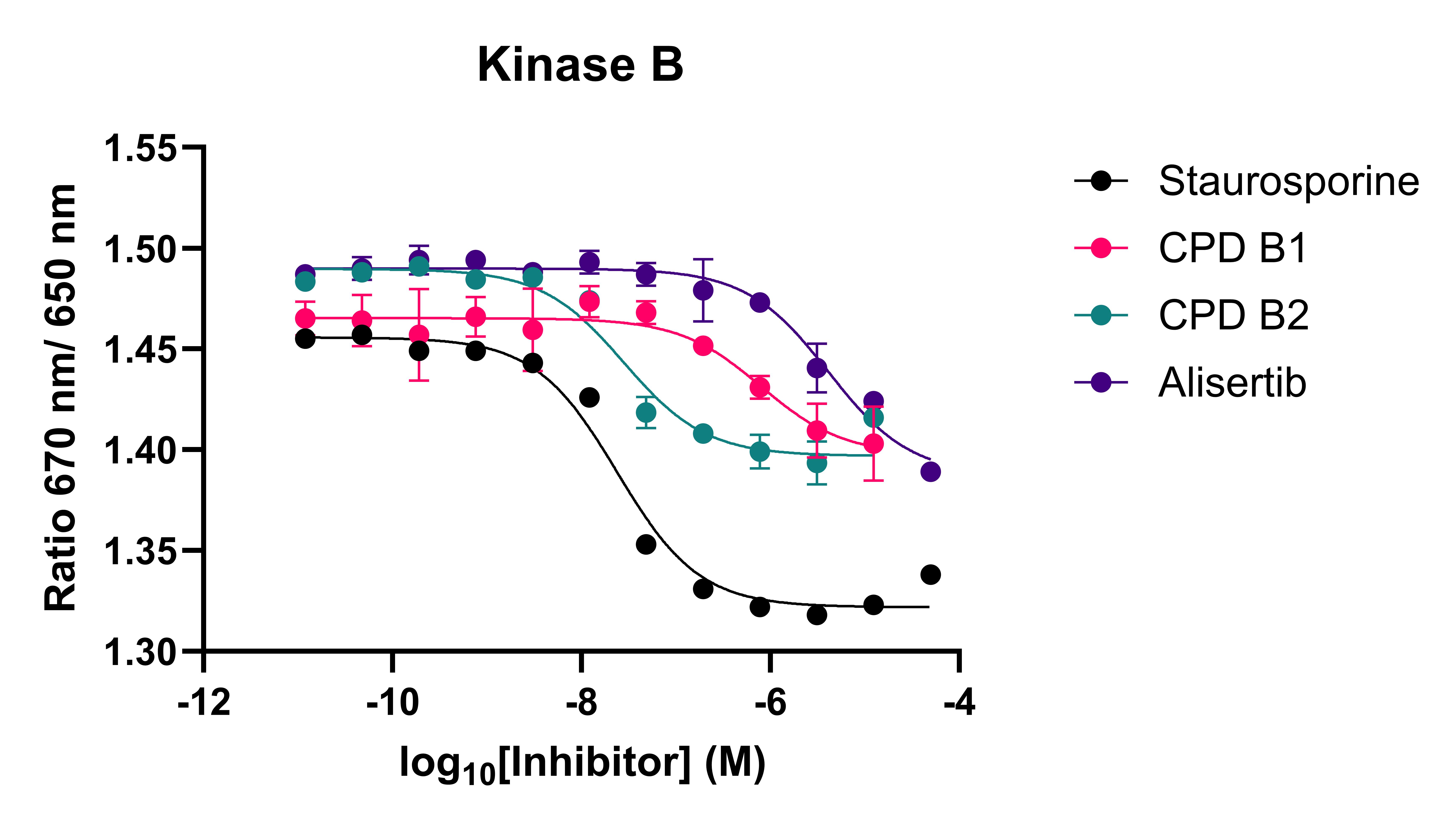
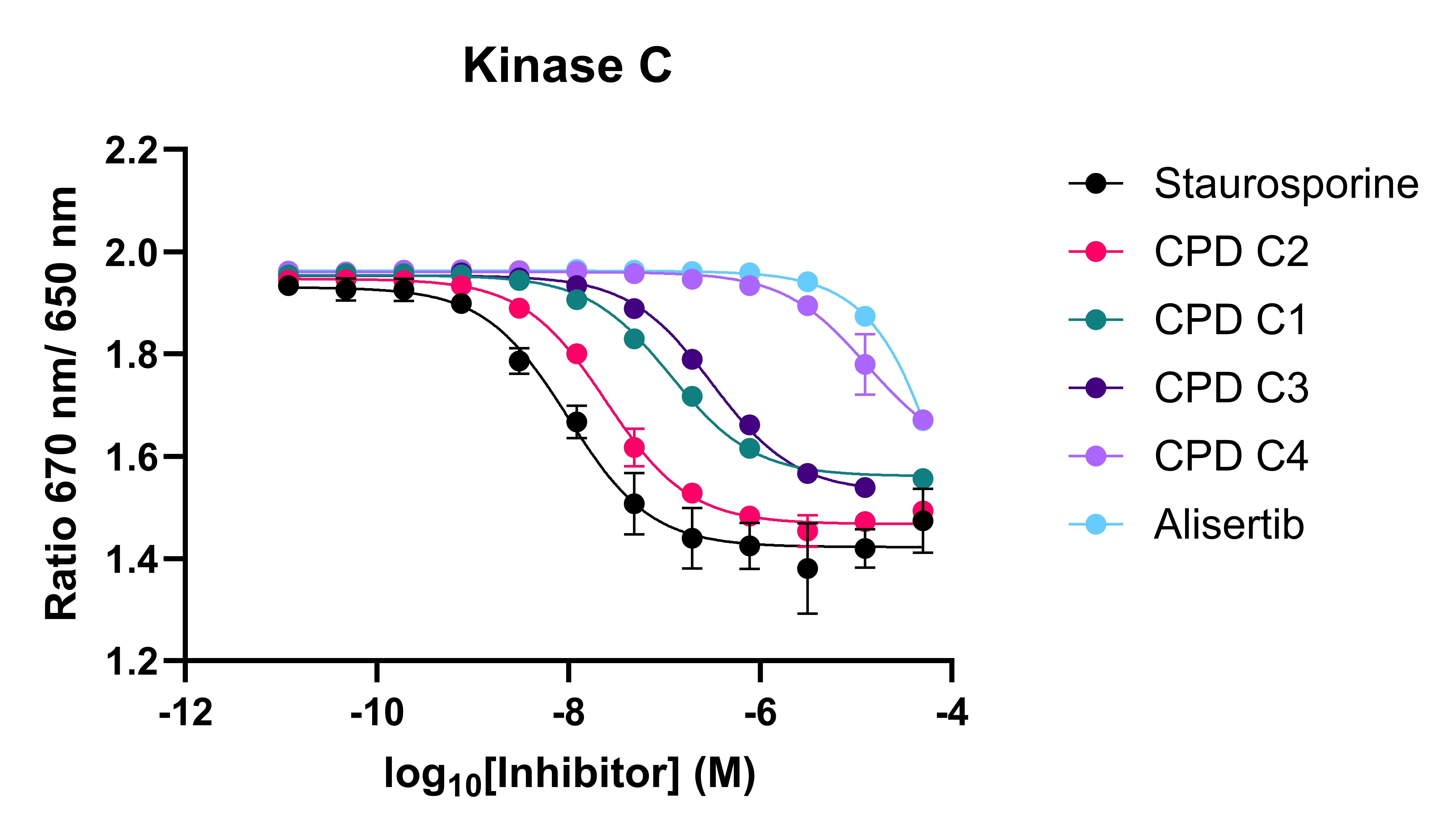
Figure 2: Spectral shift competition assay for three kinases and a panel of kinase inhibitors. CPD B1-2 and CPD C1-4 refer to specific inhibitors of kinase B and C, respectively.
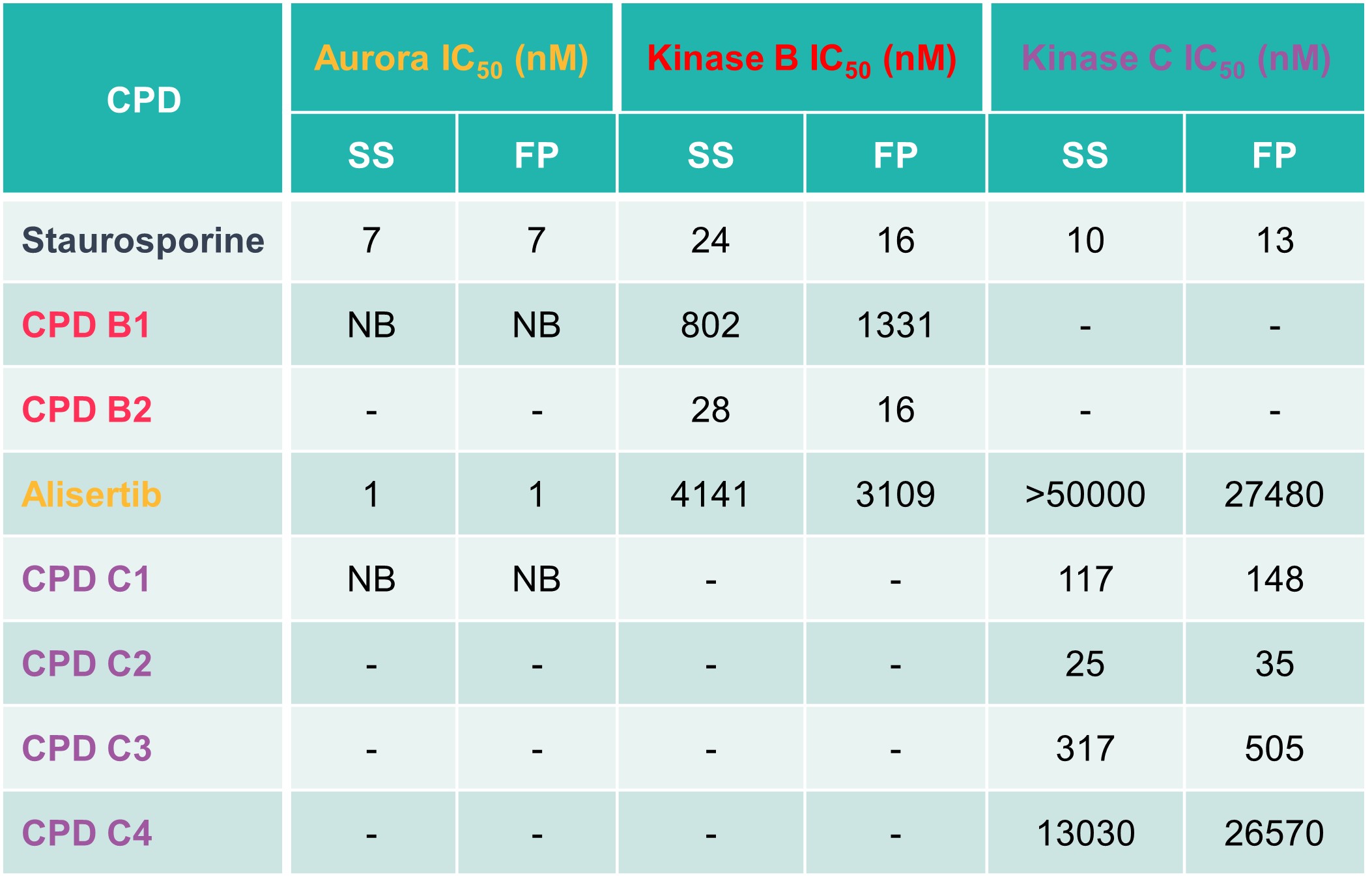
Table 2: Spectral shift IC50 values (NB = non-binder)
The spectral shift assay was able to identify both potent inhibitors and inactive compounds. The assay was also able to recapitulate selectivity data from biochemical assays, showing that data can reliably translate across assay formats, giving confidence that binding data will align with biochemical activity.
Conclusions
A competition based spectral shift assay was successfully developed for ATP-competitive inhibitors for several kinases. This assay could be applied to a broad range of kinases with minimal assay development required. Applications for this assay include medium throughput screening (MTS) against a kinase of interest or compound specificity tests against a panel of kinases.
Beyond kinases, this method is applicable to any target with a suitably labelled probe, allowing the determination of affinities of compounds for difficult to work with targets, with rapid assay development and minimal protein consumption.
Start your next project with Domainex
Contact one of our experts today