- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Neurotensin Receptor 1: Purification by PoLiPa
Generating purified, stable and functional GPCR preparations
We used the neurotensin receptor 1 (NTSR1) to demonstrate the effectiveness of our PoLiPa GPCR purification process.
Challenge
The purification of solubilised G protein-coupled receptors (GPCRs) is notoriously challenging, which limits their access to structural/biophysical techniques that can accelerate drug discovery. Techniques such as thermostabilising mutations have been used successfully in some cases, but the process is time-consuming and has to be adapted to each GPCR.
Solution
Domainex has established a generic platform to generate any purified GPCR without the need for thermostabilising mutations or detergents. This was achieved using Polymer Lipid Particle (PoLiPa) technology to stabilise GPCRs by encapsulating them in a polymer that also encloses a small disc of native cell membrane lipids.

We used the neurotensin receptor 1 (NTSR1) to demonstrate the effectiveness of the approach.
Expression
Human NTS1R was expressed in mammalian HEK293 cells. The receptor was fluorescently labelled and a 6xhis tag included. Cell surface expression was confirmed by imaging.
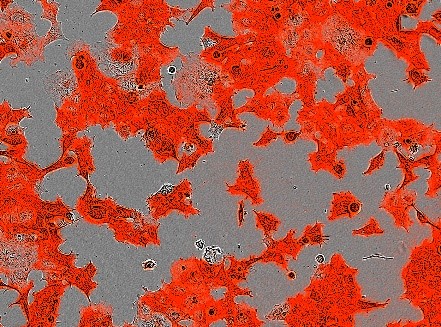
Solubilisation
Cells were solubilised with SMA2000 at room temperature. Soluble (PoLiPa containing NTS1R in native membrane) and insoluble fractions were separated by centrifugation.

Purification
Purification of the PoLiPa-NTS1R was achieved by Nickel-NTA (NiNTA) affinity, followed by size exclusion chromatography. A purity of >75% and a yield of >200µg was achieved.
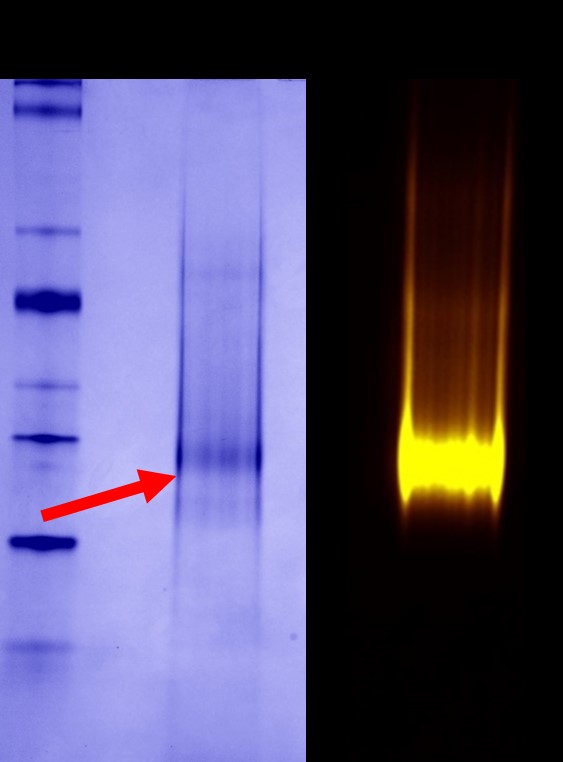
Coomassie staining showed partially purified protein (red arrow). Fluorescence detection showed a pure homogenous population of labelled receptor.
Validation
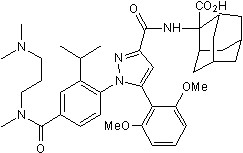
A novel ligand-observed, label-free LC-MS assay was used to pharmacologically validate the purified GPCR. A small molecule antagonist (SR-142948; Figure 5) was used as a tracer ligand. SR-142948 binding (Kd = 8.8nM) indicated that PoLiPa-NTS1R was folded and pharmacologically intact. Specific binding sites could be quantified using competition with an excess of another receptor antagonist.
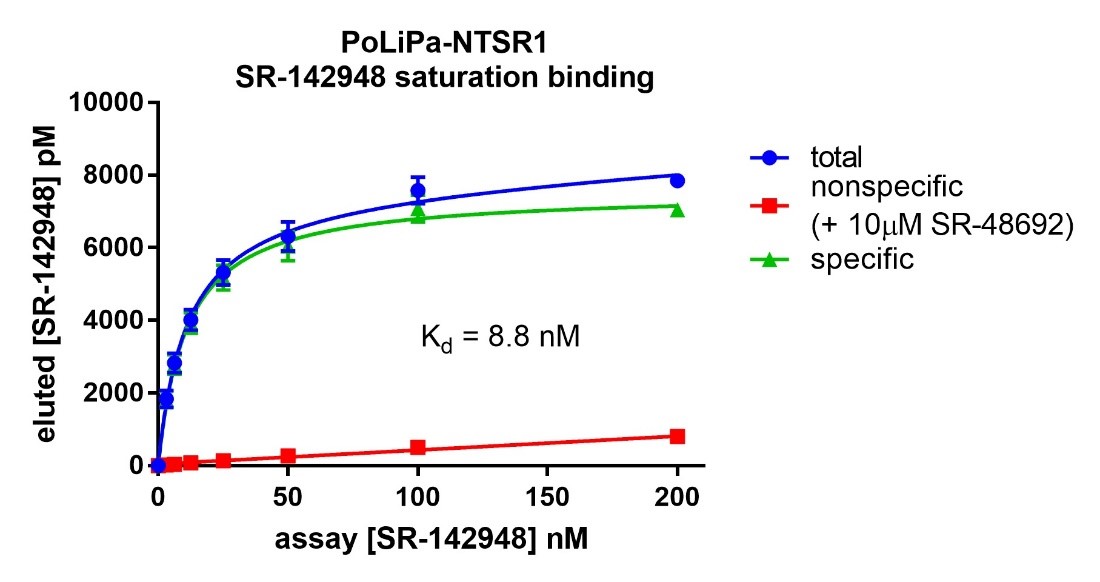
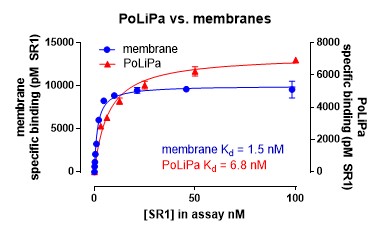
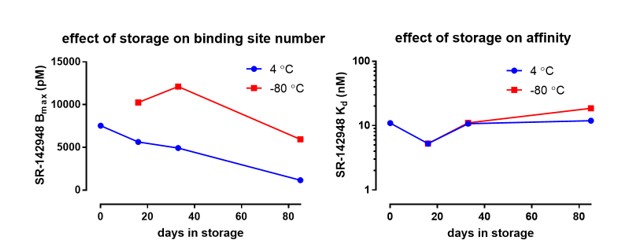
Saturation binding analysis against PoLiPa-NTSR1 was used to determine the stability during storage (Figure 8). The purified receptor was found to be very stable even with prolonged storage.
Conclusion
The PoLiPa technology developed by Domainex allows the preparation of purified soluble GPCR complexes that can be used in biochemical, biophysical and structural studies. Ligand-observed LCMS is a very useful label-free assay technique for characterising ligand-binding to PoLiPa-GPCR reagents.
The PoLiPa-GPCR reagents are very stable and can be used over a prolonged period of several months, in contrast to other preparations. This approach is fast and generic, and in these regards, is superior to methods such as detergent-solubilisation and thermostabilising mutagenesis that have been developed to achieve similar ends.
Domainex Expertise
• PoLiPa (Membrane Protein Solubilisation) • Styrene Maleic Acid Lipid Particles (SMALPs) • Bioanalytical Sciences
• LCMS Binding Assays • Protein Science • Assay Development • Cell Biology • GPCR Research
Start your next project with Domainex
Contact one of our experts today