- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Discovery Bioanalysis
At Domainex, our bioanalytical department brings over three decades of combined expertise in the quantitation of compounds in biological samples.
Within our non-regulated laboratory, our advanced suite of high-performance mass spectrometers (Waters TQ-S, TQ-XS, and G2-XS QTof) deliver state-of-the-art sensitivity, enabling detection at exceptionally low limits. Complementing our mass spec instruments, our Waters Acquity UPLC systems provide outstanding chromatographic efficiency, ensuring rapid separations and minimal data acquisition times. This powerful combination allows us to generate high-quality quantitative LC-MS data with accelerated turnaround, supporting your drug discovery programs with precision and reliability.



Our bioanalytical scientists are vastly experienced in the quantitation of standard small molecules as well as various alternative modalities, including organometallics, oligonucleotides, loaded nanoparticles, peptides and proteins. Whatever your requirements, we will collaborate with you to develop a perfectly tailored solution that meets your specific needs.
Our method establishment service allows you to utilise our expertise in developing methods for challenging compounds before transferring the method to a regulated laboratory for future use. Being a non-regulated laboratory, we can develop methods to the same scientific standards as a GLP accredited lab, but often with quicker turnaround times and without the high overhead costs associated with these labs. Once the method is developed, if required, we can perform a method establishment to ensure the method is fit for it’s intended purpose, the method can then be transferred to a regulated laboratory for use in future validation and sample analysis projects.
Our standard quantitation strategy employs targeted analysis using one of our triple quadrupole mass spectrometers. We utilise multiple reaction monitoring (MRM) data acquisition to accurately quantify the analytes of interest (Figure 1). MRM method’s are considered the gold standard approach in quantitative mass spectrometry as it provides high levels of sensitivity, reproducibility and specificity, whilst also enabling simultaneous monitoring of multiple analytes within a single injection.
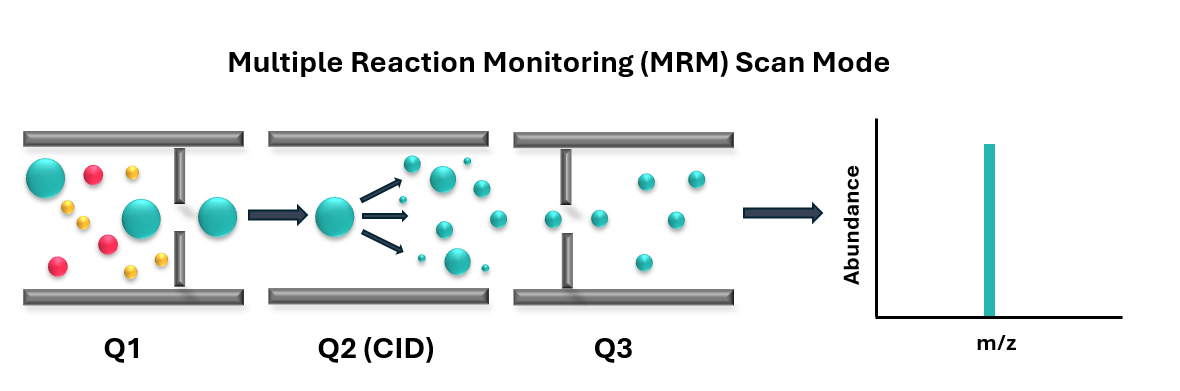
We offer several approaches for determining analyte concentrations in your samples, depending on the level of quantitation required for your project:
Qualitative Approach
Provides a comparison of analyte levels across samples without reporting exact concentrations.
Semi-Quantitative Approach
Samples are extracted alongside a solvent-based calibration curve e.g. homogenised tissue samples analysed against a calibration line prepared in buffer. This approach is quicker and cheaper than a fully quantitative approach, however matrix effects between the sample matrix and the calibration line may not be accounted for.
Quantitative Approach
Samples are extracted alongside a matrix-matched calibration curve, with an internal standard added to both samples and calibration standards. Internal standards - either analogues or stable isotope-labeled compounds - correct for matrix and volumetric effects, making this the gold standard for accurate quantitative analysis.
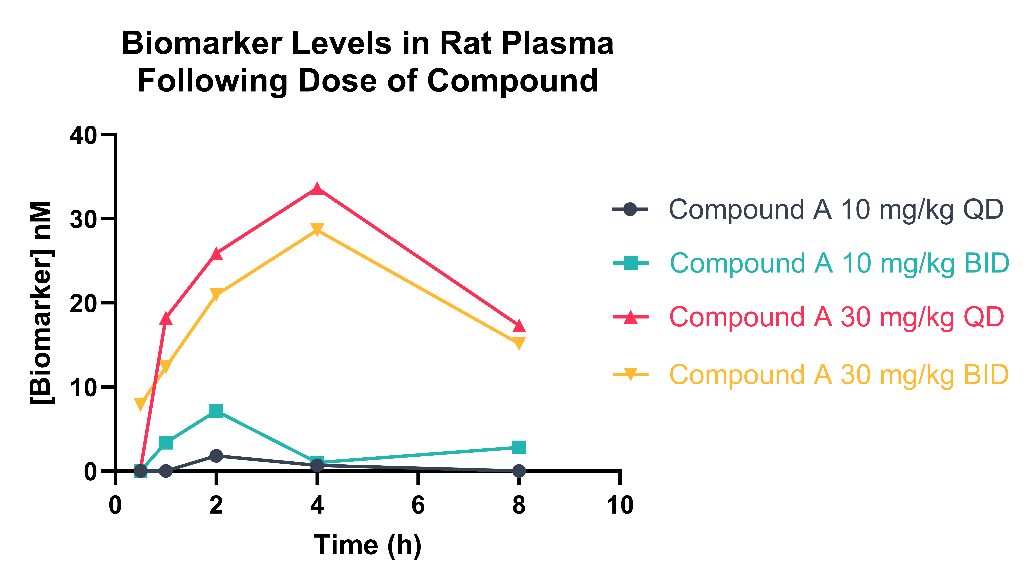
At Domainex our bioanalytical offerings extend beyond the analysis of xenobiotics, we also have the instrumentation and expertise to analyse an array of exploratory biomarkers, allowing us to provide insights into patient stratification, target engagement pharmacodynamics, and compound mechanism of action.
Our mass spectrometry and immunoassay offerings allow us to perform multiplex analysis of circulating small molecule and proteomic biomarkers, exploring the mechanisms of action, and disease response to treatment, in body fluids or tissues.
Whilst our flow-cytometry capability provides high throughput, multiparametric insights for both surface and intracellular biomarkers.
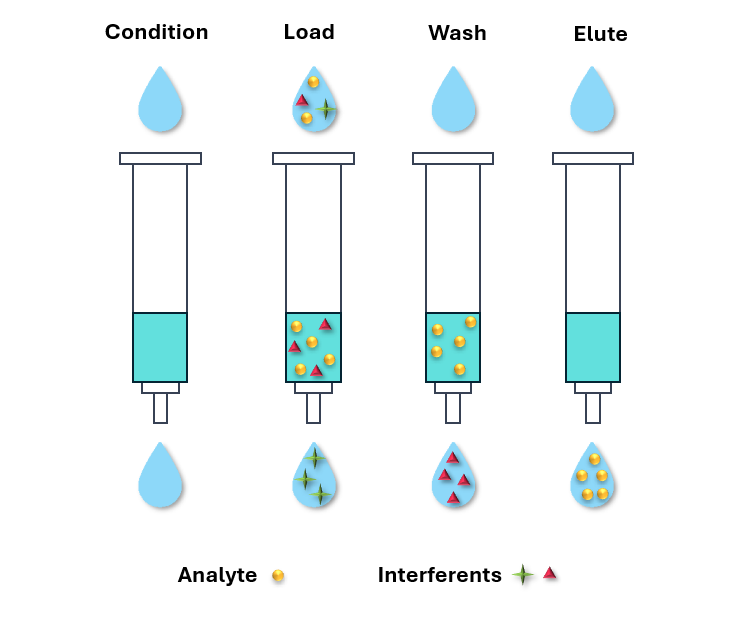
Figure 3 Schematic representation of the solid phase extraction technique for sample purification
Small Molecule Bioanalysis
Our Bioanalytical department offers extensive expertise in a wide range of sample extraction techniques for small molecules, including protein precipitation, liquid–liquid extraction, and solid-phase extraction (HLB or ion-exchange, Figure 3). The choice of method is tailored to your sample type and the detection limits required for your project. If a standard extraction approach is not suitable for your analyte(s) of interest (e.g. organometallic compounds), our experts will collaborate with you to develop a customised bioanalytical method that meets your specific needs.
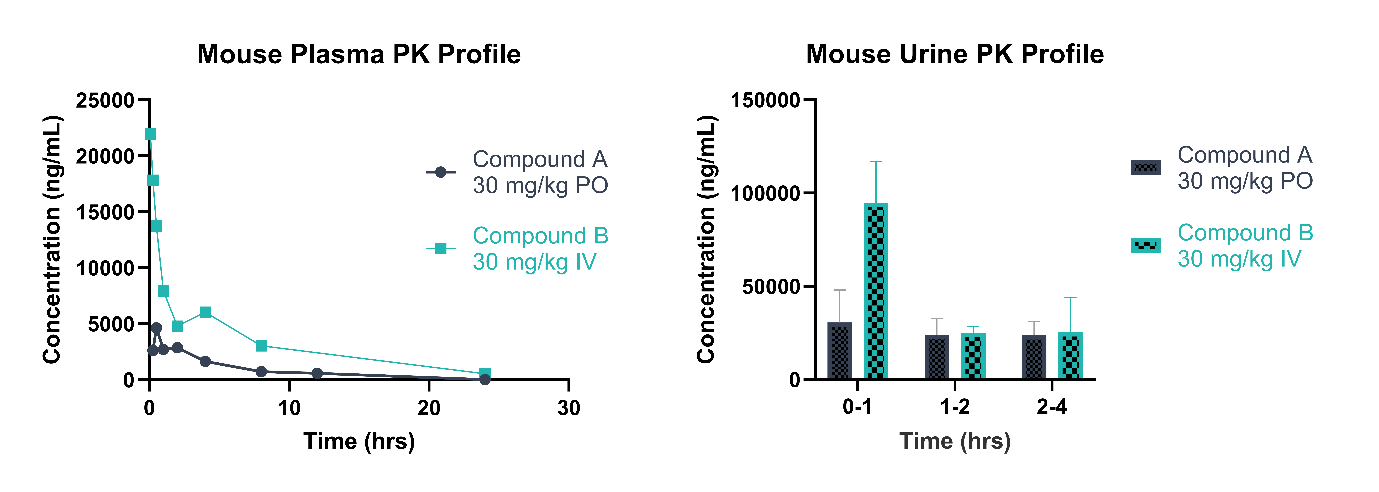
We have worked with a wide range of in vivo matrices, including - but not limited to - plasma, serum, urine, and tissues such as liver, kidney, lung, heart, muscle, and tumor (an example PK profile from a mouse study can be found in Figure 4). Our team has also analysed various ex vivo sample types, including botanical and mushroom samples.
Large Molecule Bioanalysis
In addition to our small-molecule capabilities, we also specialize in quantifying the levels large molecules within biological samples, such as biotherapeutic antibodies and proteins, as well as biomarkers. For this type of analysis, we employ a bottom-up proteomics approach (Figure 5). The bottom-up approach involves enzymatic digestion of proteins into peptides and acquisition of a surrogate peptide unique to the protein of interest. A bottom-up approach enables lower detection limits to be reached due to peptides exhibiting improved ionisation efficiency, reduced charge state distribution and improved fragmentation compared to intact proteins.
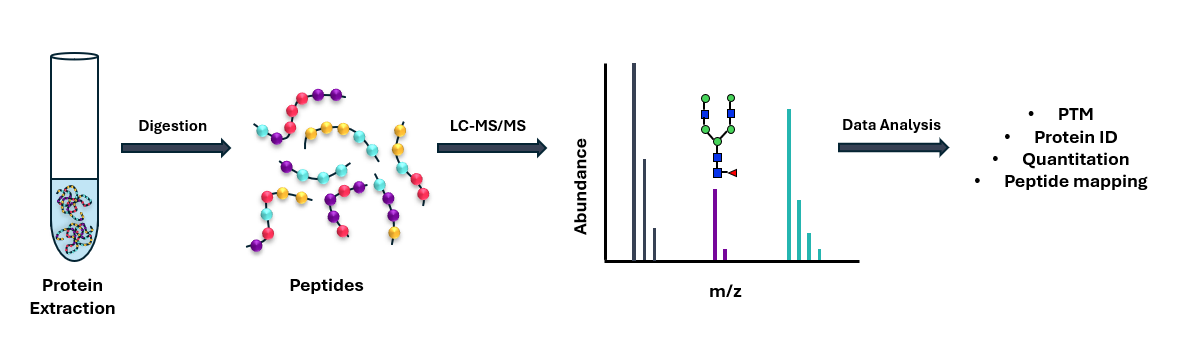
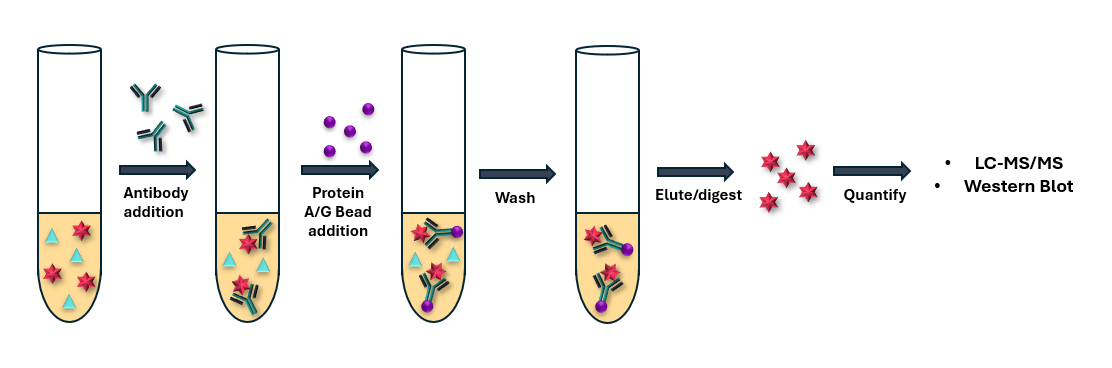
Immunoprecipitation (IP) and/or SPE techniques can also be utilised in large molecule bioanalysis as a means of achieving desired sensitivity levels. IP selectively enriches target proteins or peptides by leveraging antibody specificity, effectively removing interfering substances and concentrating the analyte of interest (Figure 6). SPE can be employed in a post-IP step or in conjunction with protein depletion to selectively retain the analyte of interest, improving chromatographic performance, matrix effects and sensitivity. Together, these techniques minimize background noise, reduce ion suppression, and increase signal-to-noise ratio, enabling accurate quantitation down to trace levels.
Method Establishment Service (Non-GLP)
A method establishment provides detailed insights into method performance and can be tailored to your requirements and gives confidence that the method will perform accurately and robustly in it’s future use. The method establishment assessments can range from a simple precision and accuracy batch to a more comprehensive, validation-like piece of work, including but not limited to:
- Inter- and intra-run precision and accuracy
- Linearity
- Selectivity
- Recovery (pooled or individual)
- Matrix effects (pooled or individual)
- Carryover assessment
- Matrix stability (room temperature, freeze/thaw cycles, and long-term storage conditions)
The entire method establishment process is fully customizable, with the ability to add bespoke assessments as needed, a method establishment report can supplied in a clear word document format.
Start your next project with Domainex
Contact one of our experts today