By the Domainex Synthesis in Review Group (Alicia Galván Álvarez, Claire Sear, Hugh Tawell, John May, Jonás Calleja Priede and Tenin Traore)
At Domainex we regularly review the literature to make sure we are aware of the latest developments and we’ve started a blog series to share our findings with you. In this, our latest blog, we have selected two recently published papers to focus on.
- Multicomponent alkene azido-arylation by anion-mediated dual catalysis. Ala Bunescu, Yusra Abdelhamid & Matthew J. Gaunt. Nature (2021)
- Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane. Patrick J. Deneny, Roopender Kumar and Matthew J. Gaunt. Chemical Science, (2021)
Get your Dopamine! β-Arylethylamine scaffolds by multicomponent dual-catalysis
The β-arylethylamine moiety is an important scaffold in biologically active small molecules, for example the important neurotransmitter dopamine is a β-arylethylamine (3,4-dihydroxyphenethylamine). However, synthesising this scaffold typically requires time-consuming and wasteful multi-step processes. In this recent publication from Matthew J Gaunt et al1 at the University of Cambridge, a single-step, multi-component approach to these scaffolds is developed, between aryl-electrophiles, alkenes, and an azide nucleophile.
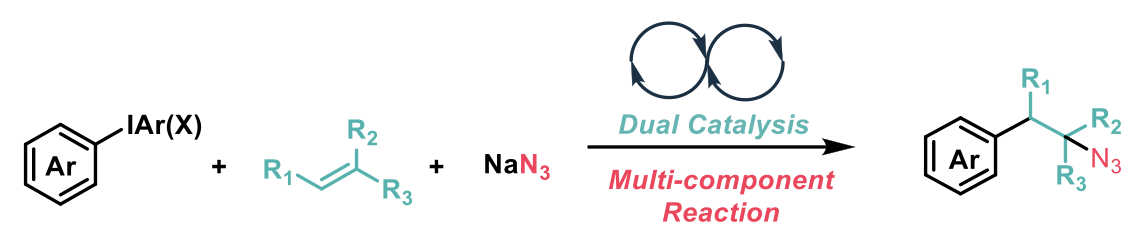
The reaction mechanism employs two discrete copper catalysts – one which acts as a photocatalyst activated by visible light, which converts the aryl electrophile to an aryl-radical. The aryl radical is then captured by the alkene. The second catalyst facilitates the group transfer process of the N-centered radical generated in the reaction, to the homobenzylic-radical, giving rise to the β-arylethylamine product.
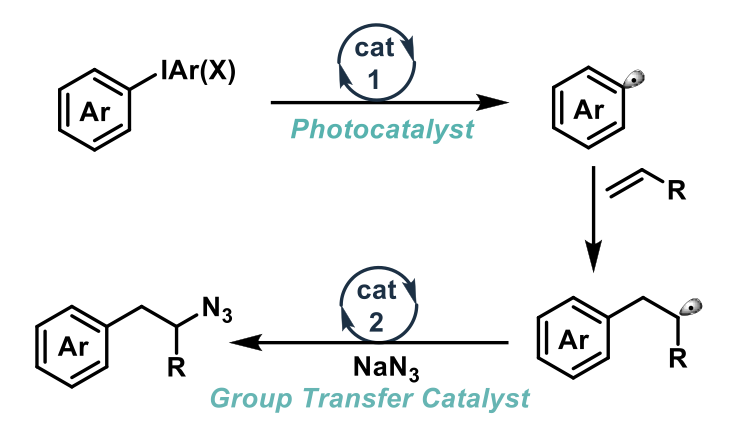
The substrate scope is impressively broad, including a number of sterically and electronically diverse alkenes. The authors also use two alkene containing natural product substrates in the reaction – isoalantalactone and (+)-nootkatone – giving good yields and chemo-selectivity over other functional groups within the scaffold, demonstrating the utility of this method, even on complex substrates. A number of diaryliodonium salts were also employed, including electron rich and poor, various substitution patterns, and heteroaromatics.
An extensive mechanistic study reveals the importance of the azide anion, not only as the nitrogen source but also in mediating the inner-sphere electron transfer in the dual-catalytic cycle.
Shining a light on physicochemical properties: Blue-light mediated monofluormethylation of imines and alkenes
Incorporation of a fluorine atom into a compound can have significant effects on both the physicochemical and pharmacokinetic properties of a compound, and therefore fluoroalkyl moieties have served as bioisosteres in the lead optimisation stage of medicinal chemistry projects.
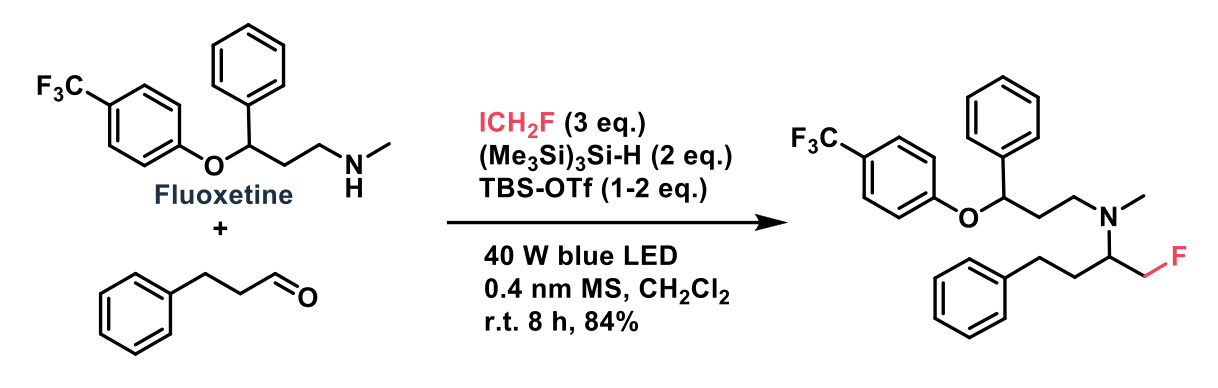
One such example is the fluoromethyl unit, a replacement for methyl and hydroxymethyl groups, potentially enhancing both metabolic stability and oral bioavailabilty. This recent Chemical Science publication from Matthew J Gaunt et al2 at the University of Cambridge describes the fluoroalkylation of iminium ions (generated in-situ from aldehydes and secondary amines, promoted by a Lewis acid) and electron-deficient alkenes, forming α-fluoromethyl amines, and monofluorinated alkanes.
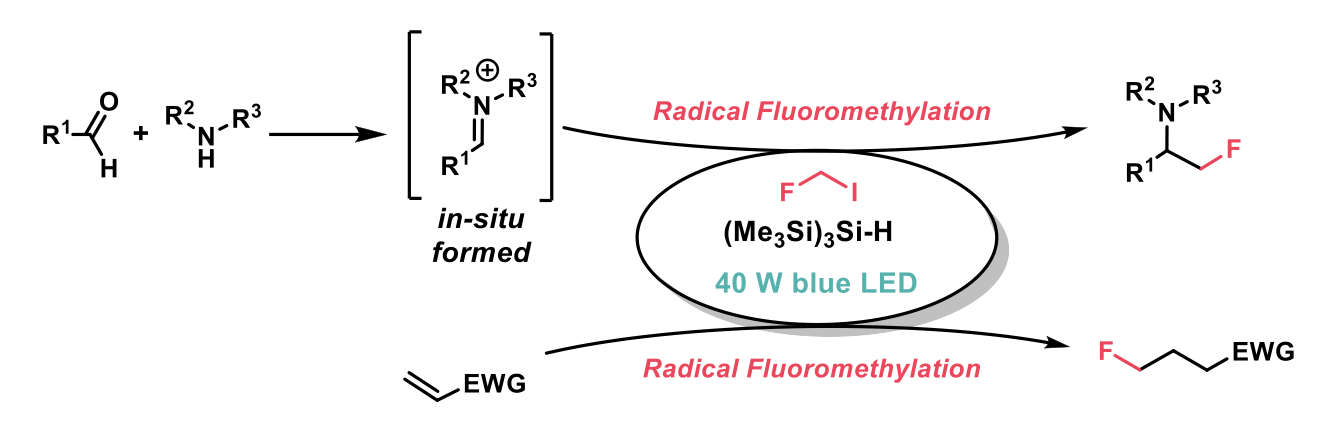
The reaction proceeds through the generation of fluoroalkyl radicals through visible-light mediated abstraction of an iodine atom by tris(trimethylsilyl)silane, followed by addition of the radical across an electron-deficient sp2 carbon centre, generating the products.
The authors point out that this modular synthetic strategy, mild reaction conditions, and wide functional group tolerance presents a greatly simplified means to access previously synthetically complex fluoroalkyl compounds. One pharmaceutically relevant example is the preparation of a fluoxetine derivative, which produced the α-fluoromethylated product in 84% isolated yield.
We hope you’ve found this interesting! If so, look out for our next synthesis review which will be available on our website soon, and get in touch to find out how we can employ the latest synthetic approaches to solve your drug discovery challenges.