By the Domainex Synthesis Group (Alicia Galván Álvarez, Claire Sear, Hugh Tawell, John May and Tenin Traore)
In the latest edition of our blog series, we have focused on the following two recent publications, which describe methods for synthesising bioisosteres:
- Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides. Juan J. Rojas, Rosemary A. Croft, Alistair J. Sterling, Edward L. Briggs, Daniele Antermite, Daniel C. Schmitt, Luka Blagojevic, Peter Haycock, Andrew J. P. White, Fernanda Duarte, Chulho Choi, James J. Mousseau and James A. Bull. Nature Chemistry, VOL 14, 2022, 160–169.
- Pd(II)-Catalyzed Enantioselective C(sp3 )−H Arylation of Cyclopropanes and Cyclobutanes Guided by Tertiary Alkylamines. Jesus Rodrigalvarez, Luke A. Reeve, Javier Miró, and Matthew J. Gaunt. JACS, 2022, 144 (9), 3939-3948.
Effective synthesis of amino-oxetanes as benzamides isosteres
The synthesis of bioisosteres is a common strategy used by medicinal chemists to improve the properties of bioactive compounds so that they can be progressed into viable drug candidates. Benzamides are extremely common pharmacophores and aryl oxetane amines represent potential bioisosteres for this category of amides, which may have improved solubility and enhanced binding to the target of interest. However, to date, aryl oxetane amines have rarely been explored due to the challenging chemistry required to synthesise them.
In this recent publication from Imperial College London, Oxford University and Pfizer Worldwide Research Development and Medical, the authors report a very effective approach for the synthesis of aryl amino-oxetanes as benzamide isosteres.1 The reaction proceeds via a sulfonyl fluoride coupling reaction with a diverse range of substituted amines under mildly basic conditions, with gentle warming.
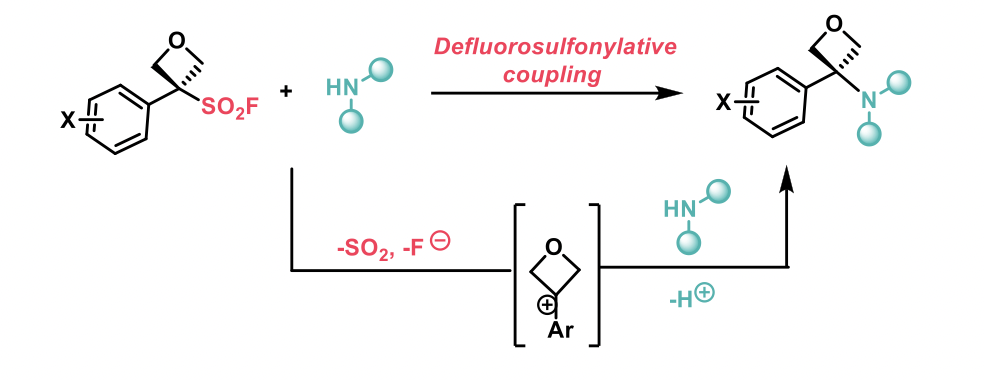
These oxetane sulfonyl fluorides undergo a defluorosulfonylation reaction rather than the SuFEx (sulfure-fluoride exchange) reaction expected for sulfonyl fluorides when in the presence with amines. The mechanism of the reaction was examined by kinetic and computational studies and was found to proceed via an SN1 mechanism due to the ability of aryl-oxetane sulfonyl fluorides to provide stable oxetane carbocations.
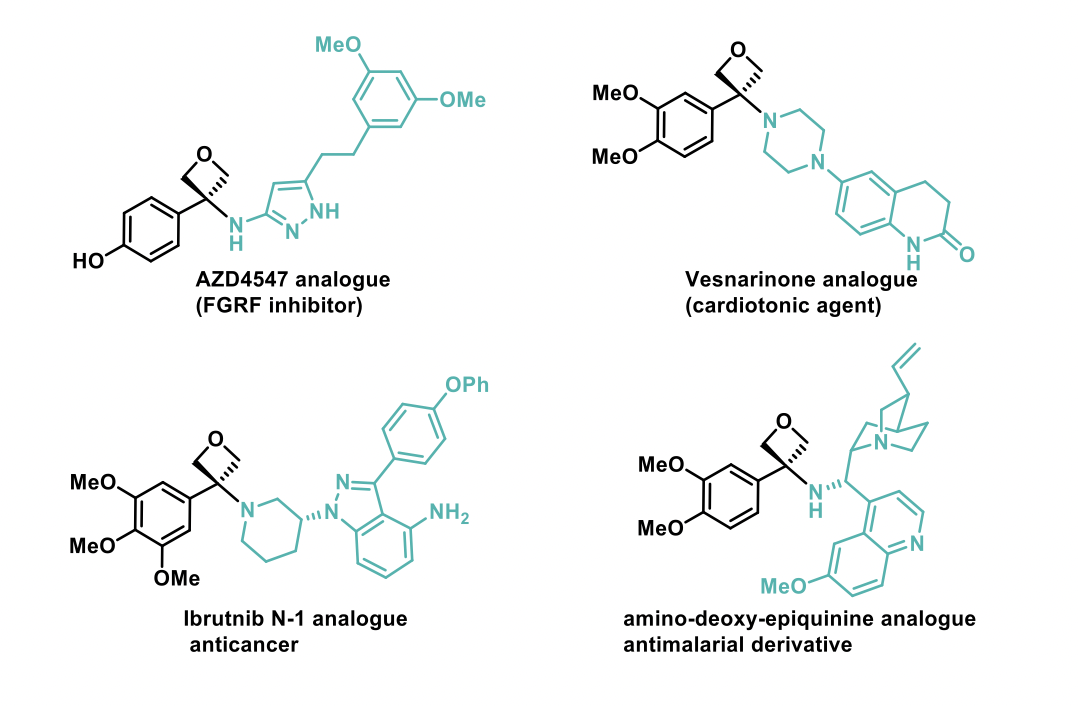
A wide range of amines could be successfully used in this reaction, including enantiopure amines, where no loss of enantiomeric excess was observed. Imidazoles and TMS-protected phenols also gave the expected desired compounds, however indoles showed reactivity through the carbon atom. The synthesis of ten oxetane analogues of benzamide-containing drugs were prepared and amongst them, examples such as the compounds shown below have been reported. This methodology was also successfully applied to array chemistry, where 25 compounds were prepared using amines with high structural diversity.
Enantioselective C(sp3)−H arylation of strained cycloalkanes
Strained cycloalkanes containing an aminomethyl moiety are commonplace in pharmaceutical candidates, agrochemicals and marketed drugs. These small polar scaffolds possess important physical and biological properties, especially cyclopropane and cyclobutene derivatives which can enhance metabolic stability and reduce lipophilicity when used as bioisosteres of groups such as phenyl or isopropyl. This work developed by the Gaunt group at the University of Cambridge reports a palladium-catalyzed enantioselective C(sp3)-H arylation of aminomethyl-cyclopropanes and -cyclobutanes with aryl boronic acids.2
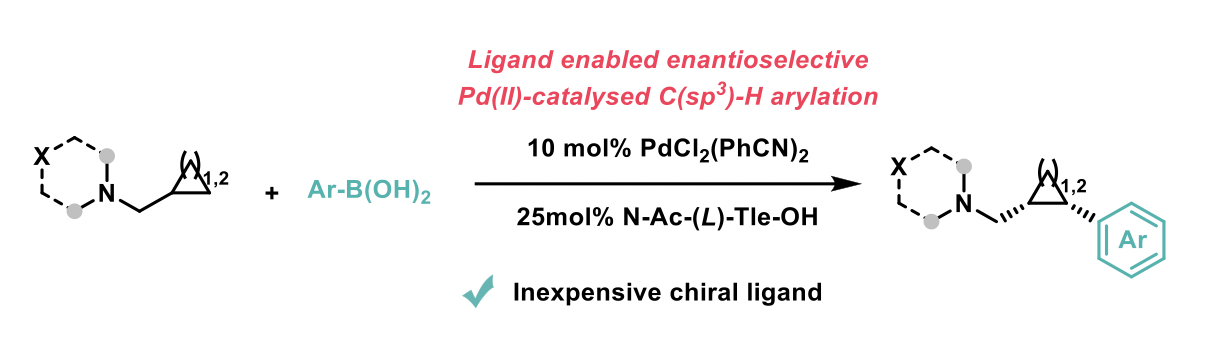
With the use of an inexpensive chiral ligand, a broad range of substrates were successfully reacted to demonstrate the scope of this methodology. This broad scope included a range of N-substituents on the aminomethyl group and a range of aryl boronic acids. The resulting product generated is the single cis diastereomer in high enantiomeric ratio (> 95:5). Computational studies were developed to predict this observed enantioselectivity.
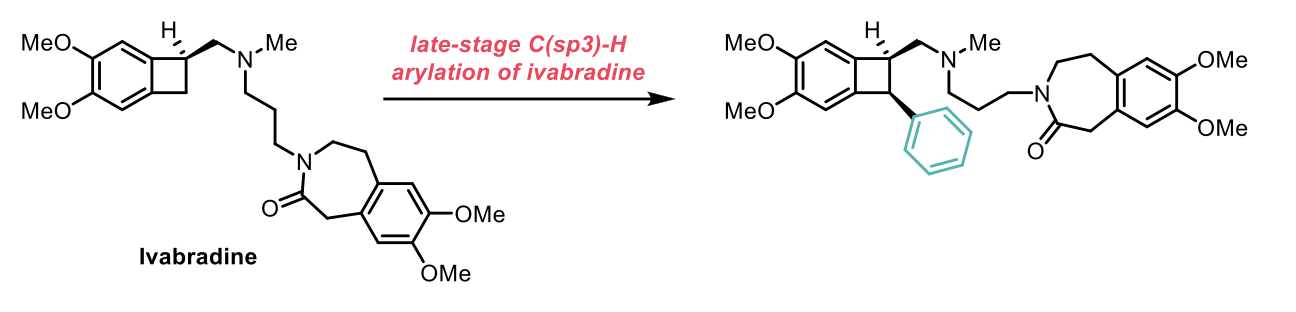
The method described is experimentally straightforward and simplifies the development of functionalized aminomethyl-strained cycloalkanes for the synthesis of biologically active small molecules, for example the late-stage C(sp3)-H arylation of ivabradine shown below.