Author: AJ Ratcliffe
Formation of glutathione (GSH) conjugates within drug candidate molecules often raises toxicological concerns that result in the redesign of the molecule to remove this liability1. Although GSH conjugates can result from direct chemical reaction of GSH, their formation is often catalysed by the glutathione S-transferase (GST) family of enzymes2. In the late 90’s researchers at Pharmacia and Upjohn identified the GSH/GST metabolic pathway as causing an unusual cleavage across the sulfonamide bond of PNU-109112, where formation of amine (1) was detected in hepatocyte incubations3.
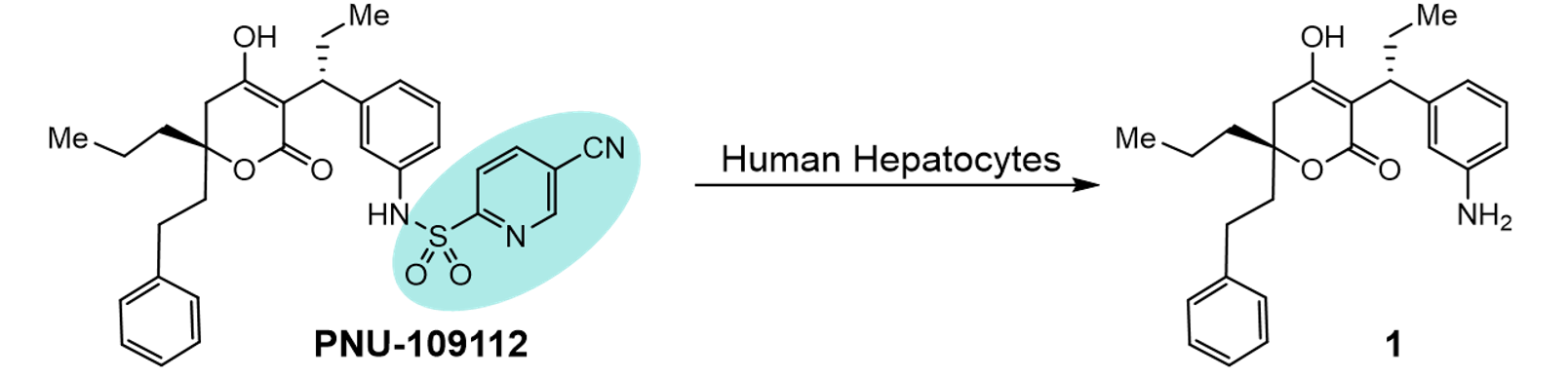
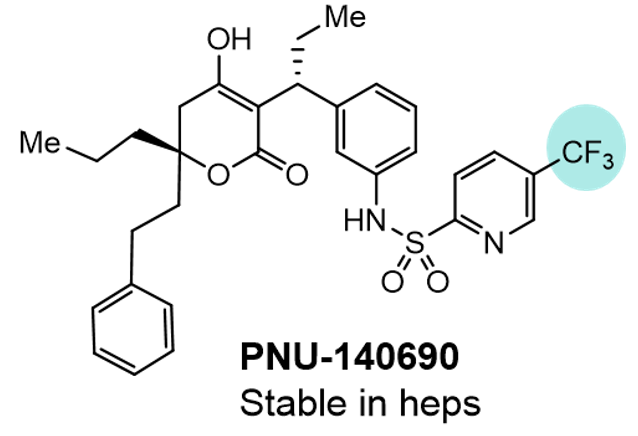
The mechanism of this cleavage is believed to be the nucleophilic addition of GSH across the S-Ar bond, resulting in the release of SO2 to give the GSH-thioether and amine 14. It is most likely that this is controlled by the electronics of the aromatic system with more electron withdrawn systems exhibiting faster cleavage, most likely conjugated in a similar manner to an SNAR substrate. However, some data does contradict this general trend as PNU-140690, a matched pair of PNU-109112 in which the para cyano is replaced with a CF3, appeared stable on incubation with human hepatocytes.
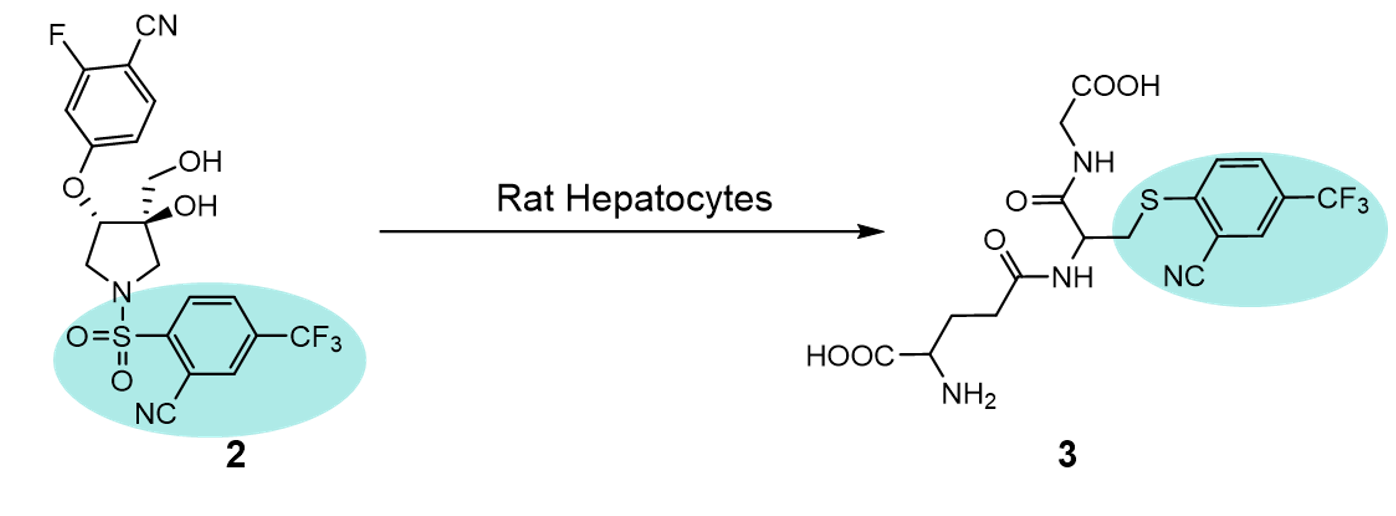
20 Years later and a similar observation of sulfonamide hydrolysis was reported in 2019 by GSK researchers. Incubation of the aryl sulfonamide transient receptor potential vanilloid-4 (TRPV4) inhibitor (2) in rat hepatocytes generated the GSH adduct (3)5. Again, one can envisage the electronics of the aryl ring playing a key role in driving the metabolic hydrolysis.
These two cases highlight that electron deficient heteroaryl/aryl-based sulfonamides may represent toxicological flags via metabolic sulfonamide hydrolysis and formation of GSH based adducts. In general sulfonamides are considered resistant to metabolic cleavage and there are countless examples of their use in drug discovery, however, it seems there are still some pitfalls to be aware of.
References
1. Monks, T, J, Toxicol. Appl. Pharma., 1990, 106, 1-19
2. Salinas, A, E, Curr. Med. Chem., 1999, 6, 279-309
3. Koeplinger, K, A et al, Drug Metab. Dispos., 1999, 27, 986-991
4. Zhao, Z et al, Drug Metab. Dispos., 1999, 27, 992-998
5. Brooks, C, A, et al, J. Med. Chem., 2019, 62, 9270-9280