By the Domainex Synthesis Group (Alicia Galván Álvarez, Hugh Tawell, Tenin Traore, Andrew Jones, Jonas Calleja, David Gibson, Andrea Bombana, James Sharpe, Venu Komanduri and Fergus Preston)
In the latest edition of our blog series, we have focused on the following two recent publications, which describe highly useful synthetic transformations including installation of C(sp3) bioisosteres and a practical method for alcohol deoxygenation transformations.
- Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres. O. Gutierrez, T Qin et. al. Nat. Chem., 2023, 15, 550–559.
- Practical and General Alcohol Deoxygenation Protocol. C. S. Yeung, Z. K. Wickens et. al., Angew. Chem. Int. Ed. 2023, 62, e202300178
Installation of C(sp3) Bioisosteres via Alkyl Sulfinates
Cyclopropyl and cyclobutyl trifluoromethyl (TF) groups have recently raised interest as bioisosteres for aromatic groups and other functionalities in drug-like molecules. These cycloalkyl groups are known to improve receptor complementarity and pharmacokinetic properties due to the increased level of saturation. Previously, alkyl sulfinate reagents have been used as radical precursors to incorporate the desired alkyl bioisosteres on functionalised heterocycles. However, the radical nature of this transformation poses reactivity and regioselectivity challenges, meaning it is not generally applicable to the functionalisation of any aromatic scaffold.
Zhou and Dykstra et al.1, have reported a method for the programmable and stereospecific installation of alkyl bioisosteres via sulfurane-mediated C(sp3)-C(sp2) cross-coupling of the respective alkyl sulfinates.
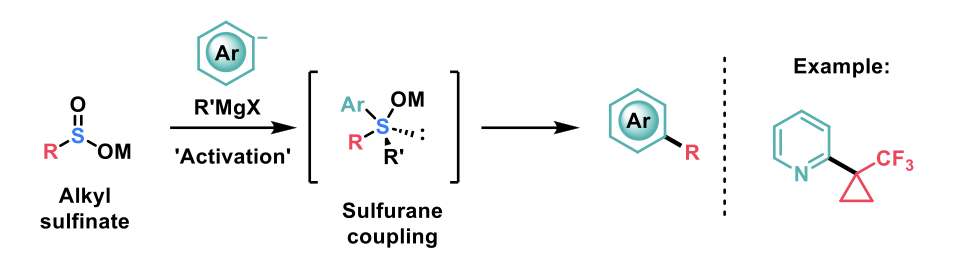
Optimal conditions for the activation of the sulfinyl intermediate were achieved using sec-butyl Grignard (s-BuMgCl·LiCl). The authors argued that this outcome resulted from a combination of nucleophilic reactivity to generate the sulfurane intermediate, and steric hindrance to promote ligand-coupling either through a three-centre concerted mechanism or 1,2-migration.
This methodology was proved successful on a broad range of TF-cyclopropyl and TF-cyclobutyl groups, as well as various substituted heterocycles. The only limitation observed for this synthetic modification was coupling with arenes containing electron-donating groups.
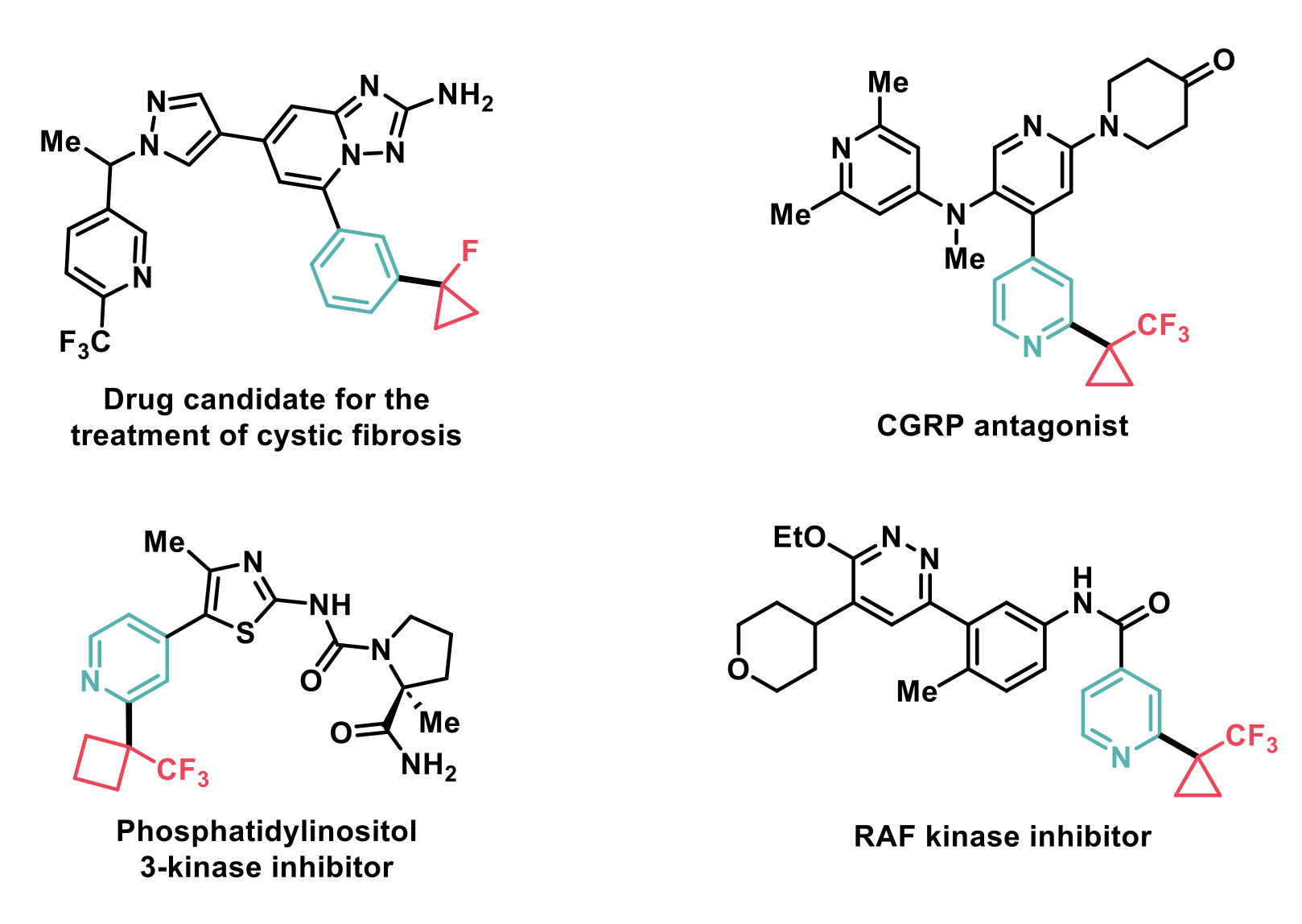
The proposed methodology was successfully applied to real-life medicinal chemistry intermediates, as shown in the figure below.
Deoxygenation: A Reductive Cleavage via Benzoate Ester Analogues
Current deoxygenation methods often rely on the use of stoichiometric trialkyl tin hydride and thiocarbonyl-based activating groups, such as in the Barton-McCombie deoxygenation reaction and its variations. Although, developments have been made in this area, the scope remains limited and the use of thiocarbonyl activating groups is still required.
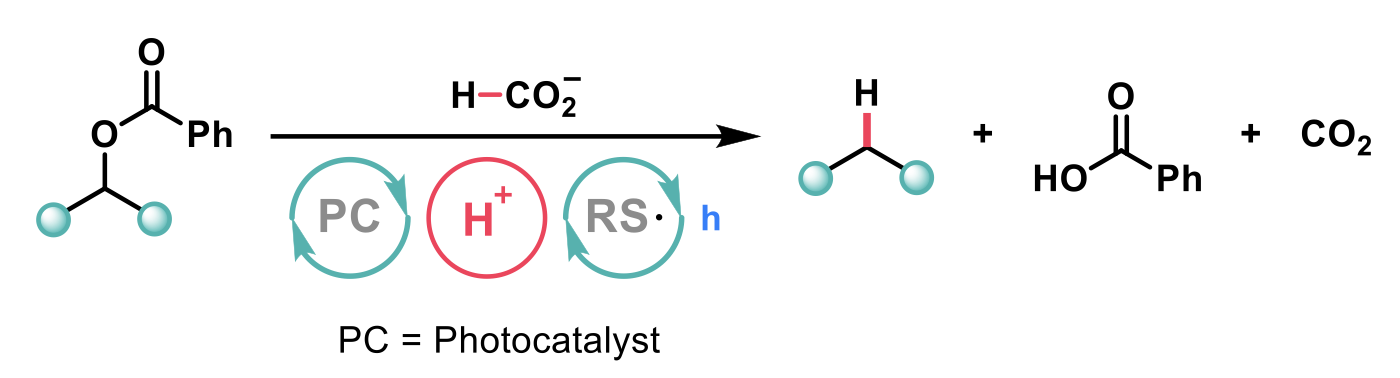
The authors, Wickens et al.2, postulated that the use of an activating group could facilitate a radical approach to the removal of the C(sp3) alcohols. Indeed, previous work has shown benzoylated alcohol derivatives can undergo C(sp3)–O bond cleavage upon single electron reduction. The authors developed a novel deoxygenation procedure by combining a photocatalytic system that generated a potent reductant from formate salts with Brønsted or Lewis acids that promoted fragmentation.
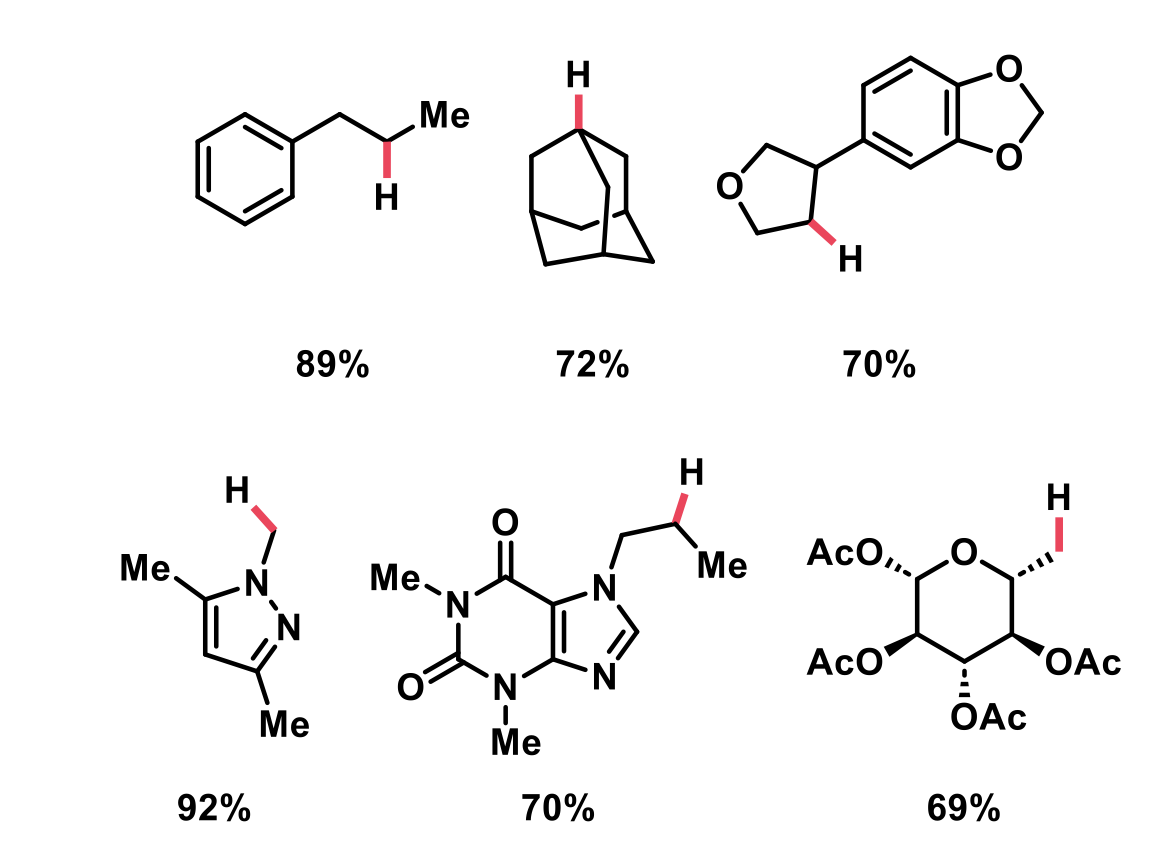
 Following optimisation of the reaction conditions, a wide range of substrates were deoxygenated. The reaction gave excellent yields for both primary and secondary alcohols, and showed tolerance to various heterocycles: furans, pyrazoles, triazoles, purines, and imidazoles. Furthermore, the presence of non-benzoylated alcohols did not interfere with the reactivity. Finally, the authors showed how their deoxygenation method can be incorporated into carbohydrate chemistry. It was found that removal of the benzoylated alcohol was selective over acetylated alcohols, even with acetylation at the anomeric alcohol.
Following optimisation of the reaction conditions, a wide range of substrates were deoxygenated. The reaction gave excellent yields for both primary and secondary alcohols, and showed tolerance to various heterocycles: furans, pyrazoles, triazoles, purines, and imidazoles. Furthermore, the presence of non-benzoylated alcohols did not interfere with the reactivity. Finally, the authors showed how their deoxygenation method can be incorporated into carbohydrate chemistry. It was found that removal of the benzoylated alcohol was selective over acetylated alcohols, even with acetylation at the anomeric alcohol.
Following their broad substrate scope, the authors demonstrated how their deoxygenation method could be incorporated into syntheses in which carbonyls are utilised, and the resulting alcohols are benzoylated and removed. The following reactions were given: ketone to methylene, aldehyde to trifluoromethyl (example shown in the scheme below), aldehyde to nitrile, and ester to cyclopropane.
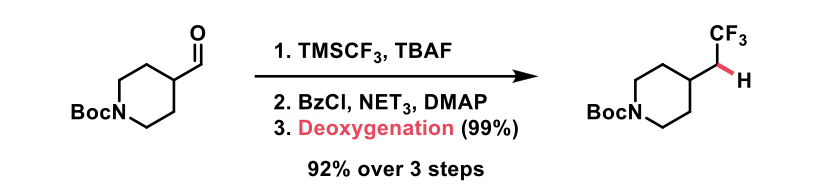
In conclusion this novel method employs mild conditions, relatively non-toxic reagents, and commercially available catalysts, to further the chemist’s toolbox across a broad substrate range. The use of benzoate esters makes this method accessible and gives only benzoic acid as a non-volatile by-product.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.