By the Domainex Synthesis Group (Alicia Galván Álvarez, Hugh Tawell, Tenin Traore, Andrew Jones, David Gibson, Andrea Bombana, James Sharpe, Venu Komanduri, Fergus Preston, Brahmam Medapi and Robyn Presland).
In the latest edition of our blog series, we have focused on the following two recent publications, which describe highly useful synthetic transformations:
- 2-Oxabicyclo[2,1,1]hexanes as Saturated Bioisosteres of the ortho-Substituted Phenyl Ring Pavel K. Mykhailiuk et. al., Nat. Chem., 2023, ASAP.
- Tunably strained metallacycles enable modular differentiation of aza-arene C–H bonds. Shi et al, Nat Commun., 2023, 14, 3986.
A new ortho-Phenyl Bioisostere
The ortho-substituted phenyl motif is found in many drugs and agrochemicals. Bioisosteric replacement has traditionally focused on carbocycles such as cyclopropyl, cyclopentyl, and cyclohexyl rings.
![synthesis of 2-oxabicyclo[2.1.1]hexanes](/sites/default/files/inline-images/Screenshot%202023-09-06%20at%2009.59.00.png)
Mykhailiuk et al1 disclose the synthesis of 2-oxabicyclo[2.1.1]hexanes, demonstrating improved physicochemical properties. Furthermore, X-ray crystallographic studies have shown 2-oxabicyclo[2.1.1]hexanes to have similar geometry when compared to the phenyl counterpart. In addition, 2-oxabicyclo[2.1.1]hexanes are less prone to ring-opening by nucleophiles and more stable compared to 2-oxabicyclo[1.1.1]pentanes.
![2-oxabicyclo[1.1.1]pentanes](/sites/default/files/inline-images/Screenshot%202023-09-06%20at%2009.59.19.png)
The 2-oxabicyclo[2.1.1]hexanes were synthesised via a photochemical, intramolecular [2+2] cycloaddition of an oxygen tethered diene with an ester substitution. Saponification of the crude ester yielded the acid as a crystalline solid upon recrystallisation. The diene precursor required for cycloaddition can be synthesised via a high yielding, two-step synthesis, starting from commercially available propargyl alcohol. The authors1 demonstrated the applicability of this route to scale-up chemistry by performing the reaction on a 10 g scale. Furthermore, the photocyclisation method was compatible with various substituents on the aromatic core, such as alkyl groups, fluorine and chlorine atoms, methoxy and trifluoromethyl groups, as well as various substituted pyridines.
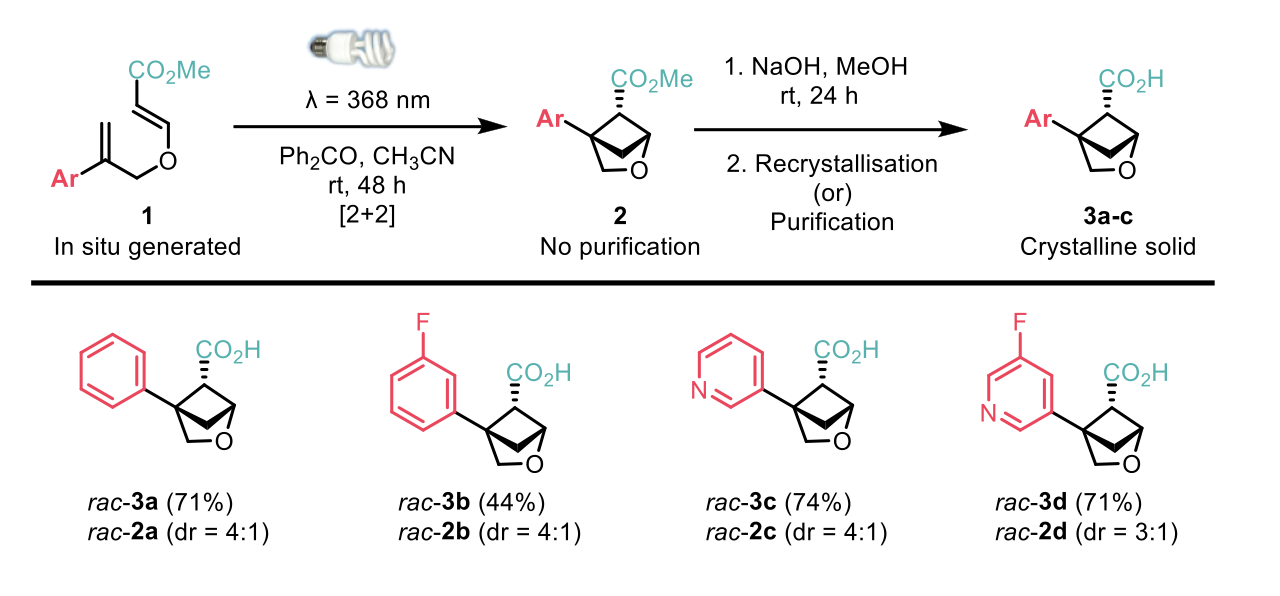
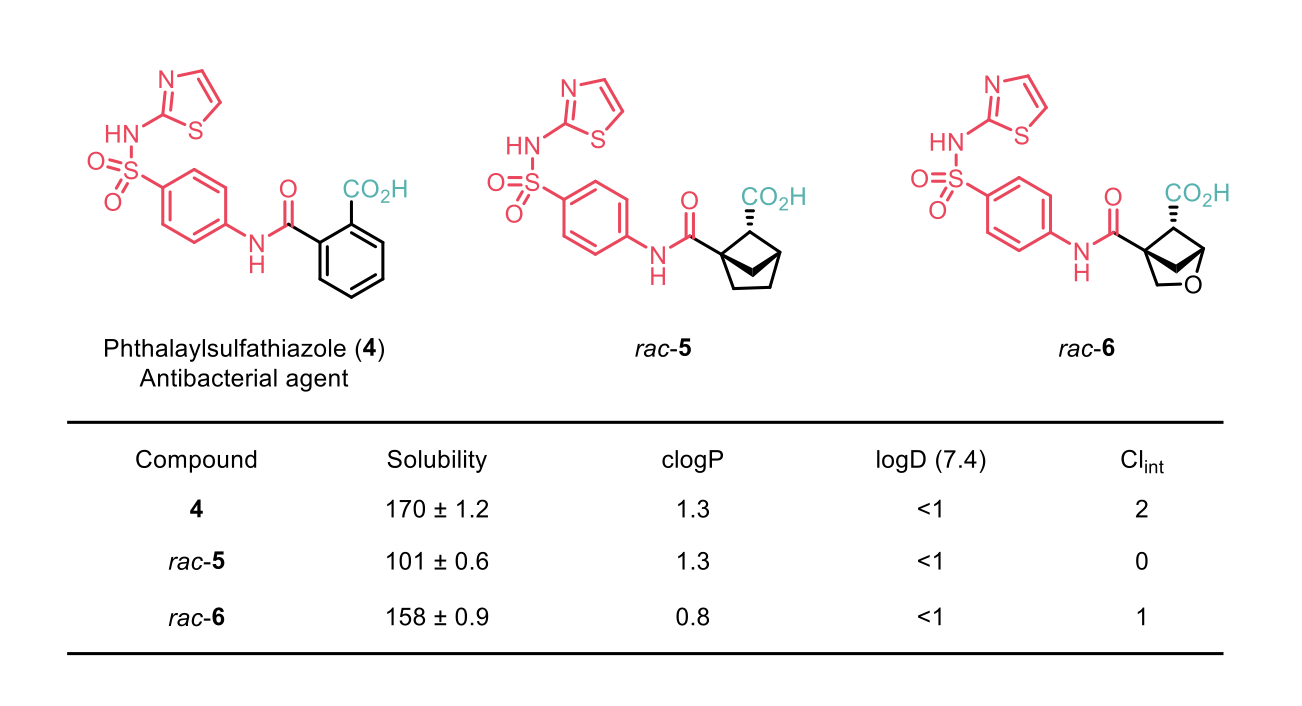
Mykhailiuk et al1 also demonstrated post-functionalisation of the 2-oxabicyclo[2.1.1]hexanes to synthesise bioisosterically modified drugs such as Phthalylsulfathiazole, an antibacterial agent. Comparison between Phthalaylsulfathiazole and the bioisosterically modified drug showed improved physiochemical properties.
Site Selective C-H activation
C-H activation methodology has been widely used over the past decades to functionalise carbon centres in an atom-economical and streamlined way. However, due to the presence of several C-H bonds having similar strengths and steric environments, achieving regioselectivity has always been a challenging endeavour. Obtaining highly strained, three- and four-membered metallacycles via directed C-H activation remains very challenging.
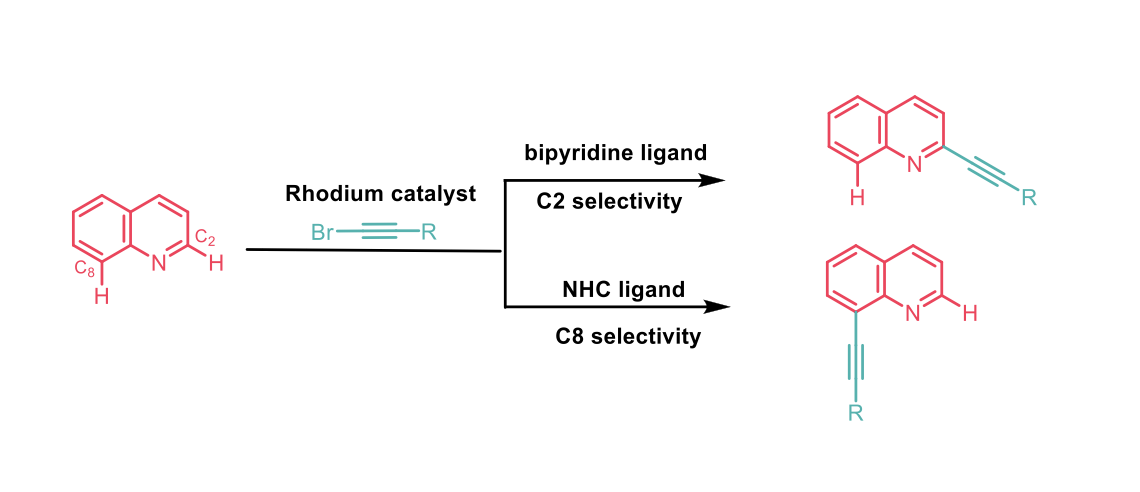
To this end, Shi and co-workers2 report a successful strategy for the regio-divergent C-H activation of aza-arenes enabled by tuneable, strained metallacycles, under monomeric rhodium catalytic conditions. The differentiation between two C-H bonds proceeds through a switchable three- or four-membered-ring cyclometallation pathway, determined by the structure of the ligands. A bipyridine-type ligand gave a three-membered metallacycle, whereas an N-heterocyclic carbene (NHC) ligand favoured the generation of the four-membered metallacycle.
The generality of this method was demonstrated with a range of aza-arenes, such as quinoline, benzo[f]quinolone, phenanthridine, 4,7-phenanthroline, 1,7-phenanthroline and acridine.
Specific attention and work were conducted towards a methodology for the incorporation of alkyne motifs (using bromoalkyne) into aza-arene compounds to provide a versatile handle for further modifications. A range of electron poor and electron rich quinoline’s successfully underwent this transformation with good to excellent yields (43-96%), and very good regioselectivity ratio between C2 and C8, depending on the ligand used. The procedure was tolerated with linear, bulky, or sterically hindered bromoalkynes.
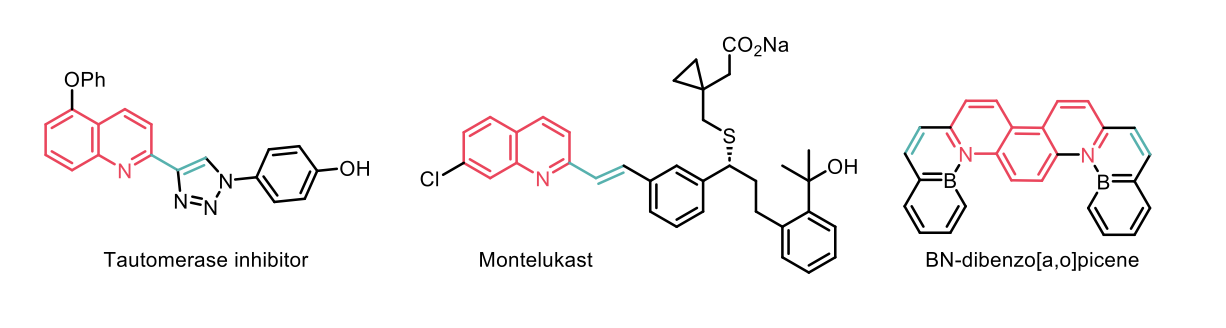
The authors were able to construct more complex compounds, especially pharmaceutically relevant ones; as well as obtain functionalised boron-containing organic compounds for material applications, thus demonstrating further synthetic utility and application of this method.
In conclusion, the work highlighted a very powerful and selective C–H activation methodology through a benzo-fused three- or four-membered ring cyclometallation pathway determined by the ligand used. This strategy also offers the potential for further editing of aza-arene C–H bonds, for the design of new more complex bioactive molecules, natural products and functional materials.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.