By the Domainex Synthesis Group (Alicia Galván Álvarez, Hugh Tawell, Tenin Traore, Andrew Jones, Jonas Calleja, David Gibson, Andrea Bombana, James Sharpe, Venu Komanduri and Fergus Preston)
In the latest edition of our blog series, we have focused on the following two recent publications, which describe highly useful synthetic transformations:
- Rapid Access to 2-Substituted Bicyclo[1.1.1]pentanes, David W.C. MacMillan et. al., J. Am. Chem. Soc., 2023, 145, 3092-3100
- Synergism of Fe/Ti Enabled Regioselective Arene Difunctionalisation, Xin-Fang Duan et. al. J. Am. Chem. Soc. 2023, 145, 1542-1547
Rapid Access to 2-Substituted Bicyclo[1.1.1]pentanes
Bicyclo[1.1.1]pentanes (BCPs) have been widely used as a bioisostere replacements for substituted arene rings in lead pharmaceutical candidates. The replacement often results in improved properties such as increased solubility, greater metabolic stability, and decreased non-specific binding. Whilst there has been significant adoption of 1,3-substituted BCPs in medicinal chemistry programmes, the corresponding 2-substituted BCPs remain underrepresented. The current approaches to 2-substiututed BCPs rely on installation of the bridge substituent prior to BCP core construction, resulting in lengthy synthetic routes.
MacMillan and co-workers1 have recently reported methodology involving C–H functionalisation of the BCP core to yield 2-bromintated BCPs which were further functionalised via metallophotoredox-mediated arylation with a range of aryl bromides (see scheme below).
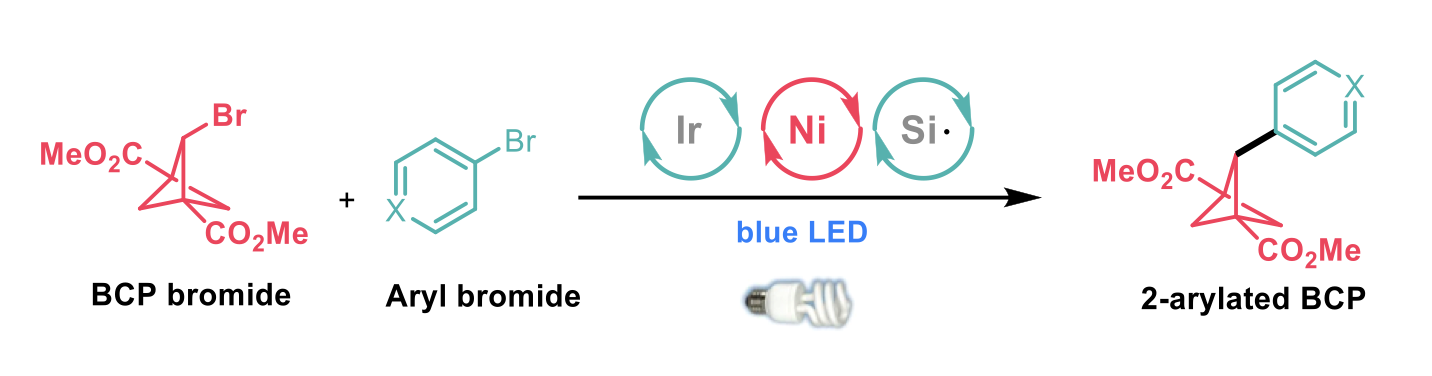
Using solvents which lack weaker, hydridic C–H bonds, favoured arylation over protodehalogenation. These conditions were tolerated with electron-deficient and electron-rich substituents in the para position on the aryl ring. Furthermore, methyl esters at the ortho-, meta-, and para positions were well tolerated. The conditions were applicable to sensitive functional groups such as tertiary amines, carbamates, nitriles, and aryl chlorides. The protocol was also applicable to a range of five- and six-membered heterocycles as well as fused heterocycles.
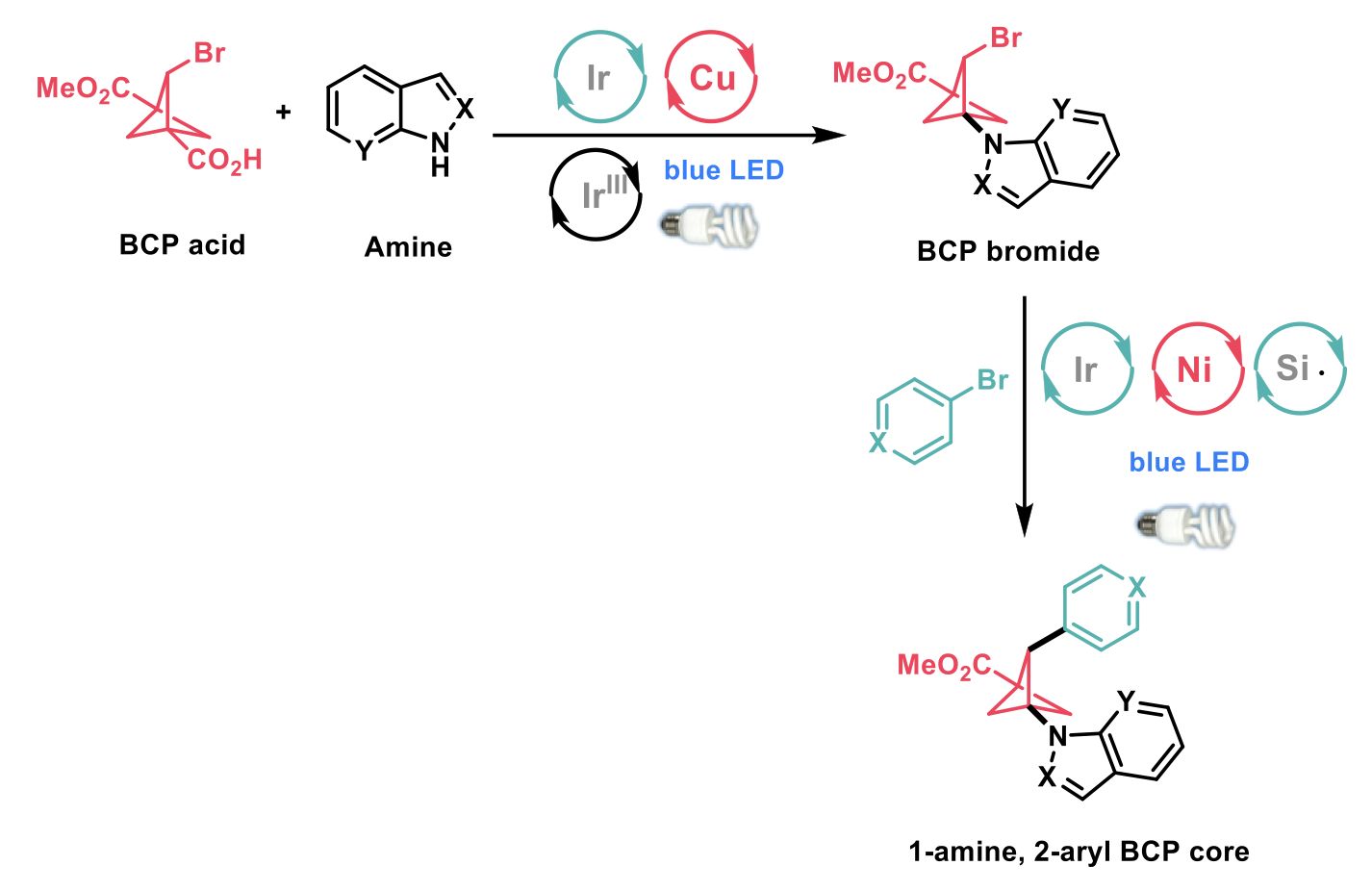
The authors showed further utility of this approach by performing sequential photoredox-mediated functionalisation reactions, employing decarboxylative amination of the bridgehead carboxylic acid, followed by metallophotoredox silyl-radial cross-electrophile coupling to furnish 1-amine, 2-aryl BCP cores (as shown in the scheme below). This protocol allowed for the introduction of hetero(aryl) bromides, including indoles, pyridines, quinolines and unprotected carbamates in moderate to excellent yield (45-90%).
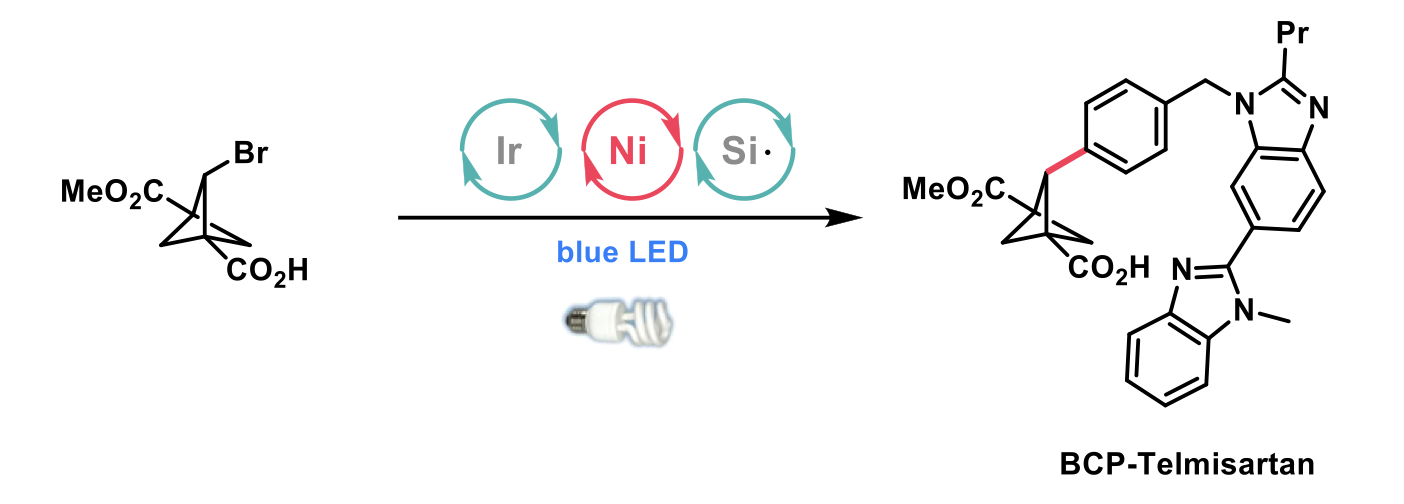
The authors applied the metallophotoredox-mediated arylation to a range of pharmaceutical analogues, including the synthesis of BCP-telmisartan in two steps with 63% yield. Previously, the best synthesis reported was completed in 10 steps with an overall yield of 10%.
The BCP pharmaceutical analogues were tested in comparison to their aryl-containing equivalents, showing improved or similar lipophilicity, solubility and Experimental Polar Surface Area (EPSA), demonstrating the potential of BCP analogues to improve the pharmacological properties of drug candidates.
For further reading, you can also check out our related, previous blog about bicyclo[3.1.1]heptane (BHPs) as bioisosteres for meta-substituted benzenes: Synthesis in Review: Bicyclo[3.1.1]heptanes (BCHeps) as bioisosteres for meta-substituted benzenes | Domainex
Synergism of Fe/Ti Enabled Regioselective Arene Difunctionalisation
A method to successfully carry out regioselective ortho-difunctionalisation (ODF) of arenes, with application in the synthesis of natural products, functional materials and pharmaceuticals is highly desirable, and represents a long-standing challenge in organic chemistry. So far, previous work published in the field has utilised aryne chemistry or Catellani-type reactions with some success, but challenges remain, such as the preparation of the bimetallic reagents or obtaining regioisomer mixtures of the final desired product.
The work reported by Duan et al 2 (depicted in the scheme below) successfully demonstrates that regioselective ODF can be performed via a key 1,2 arylene Fe/Ti heterobimetallic intermediate (enabled by Fe/Ti synergistic interaction), with the regiocontrol arising from the relative positions of the Ti and Fe, and not from the actual directing groups.
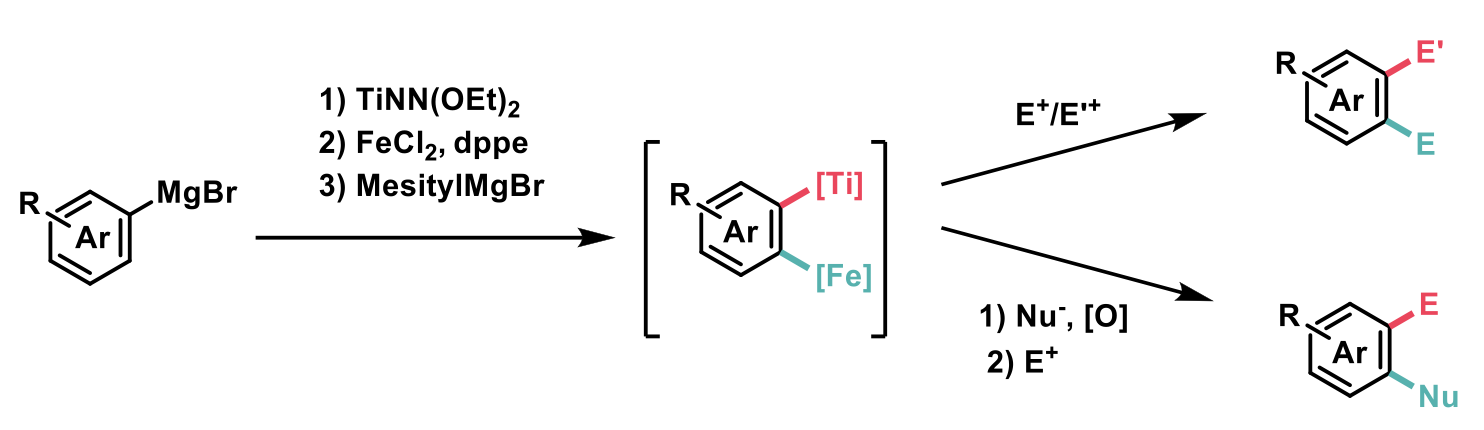
In order to identify final optimised conditions, a series of ligands were screened and Me2NCH2CH2N(CH2CH2OH)2 (abbreviated HNN) was identified as the optimal Ti ligand and afforded the highest yield of 67% for the desired compound in a model reaction. The presence of the NMe2 group was believed to be vital in the role of bridging Ti and Fe. Alternative ligands screened hardly afforded the desired product. Increasing steric hindrance as well as decreasing the electron density around the nitrogen atom of the ligand resulted in no reaction. The authors showed that MesitylMgBr is essential to promote the deprotonative formation of the key Fe/Ti synergetic intermediate. Reactions carried out without the presence of a stoichiometric amount of 1,2-bis(diphenylphosphino)ethane (dppe) resulted in lower yields, the role of the dppe being to assist the ferration.
Experiments comparing the reactivity of C-Fe with C-Ti bonds indicated that the Fe site reacts preferentially over the Ti site, this difference in reactivity resulted in high regioselectivity. In cases where the ferration could potentially take place with two C-H bonds ortho to the C-Ti bond, the steric bulk of the alkyl/aryl substituent would govern the regioselectivity and the reaction would occur at the less hindered site. Interestingly, Cu(I) salts were required for alkylation, acylation, amination, and phosphorylation at the Ti site; and for acylation and phosphorylation at the Fe site.
Several substrates were successfully converted using this procedure to afford the ortho-dicarboxylic acids, aryl Grignard reagents and halogenated substrates. Anthranilic acids were also easily accessed in a one-pot manner, a marked improvement on the usual multistep transformations.
Represented below are a selection of successful examples obtained using this methodology including valuable compounds derived from natural products, for pharmaceutical applications, and also diversification of late-stage pharmaceuticals.
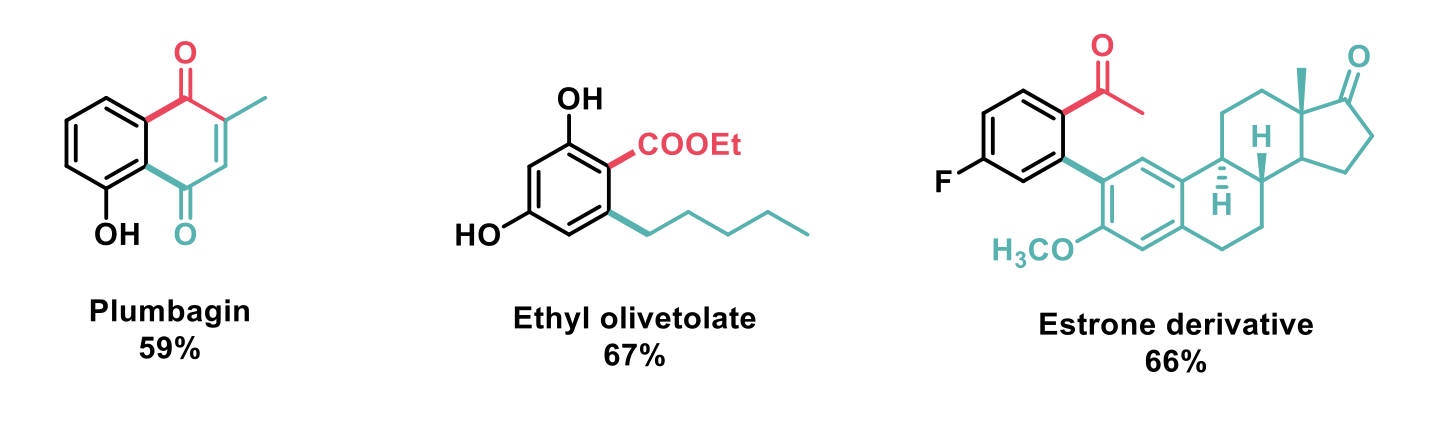
In summary, the work outlined here has demonstrated the feasibility of access to highly regioselective E/E’ and Nu/E type ODF reactions. This novel and widely applicable approach has allowed the synthesis of non-symmetrical, highly functionalised and diversified substrates with good yields.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.