- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
MDCK Permeability Assay
Domainex’s MDCK assay provides a robust in vitro model for evaluating passive permeability and transporter-mediated efflux, critical parameters in predicting oral absorption, brain penetration and pharmacokinetic behaviour of test compounds.
Utilising differentiated Madin-Darby Canine Kidney (MDCK) cells engineered to express clinically relevant transporters such as P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP), this assay enables precise characterisation of compound permeability and identification of efflux liabilities before they become costly setbacks.
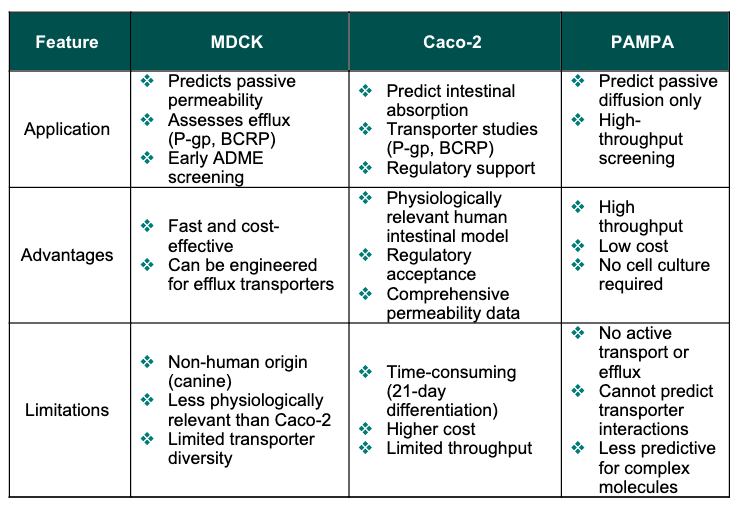
Similar to the Caco-2 permeability assay, the MDCK cell layer separates apical and basal compartments, allowing the assessment of both apical-to-basal (A-B) and basal-to-apical (B-A) transport (Table 1). This bidirectional setup enables both evaluation of passive diffusion and identification which compounds are substrates for efflux transporters, providing critical insights into potential bioavailability issues and drug-drug interaction risks. The MDCK assay offers additional advantages, including lower costs and faster turnaround times compared to Caco-2 assessment, making it an especially valuable tool for early ADME profiling and lead optimisation.
Domainex’s Standard Experimental Procedure:
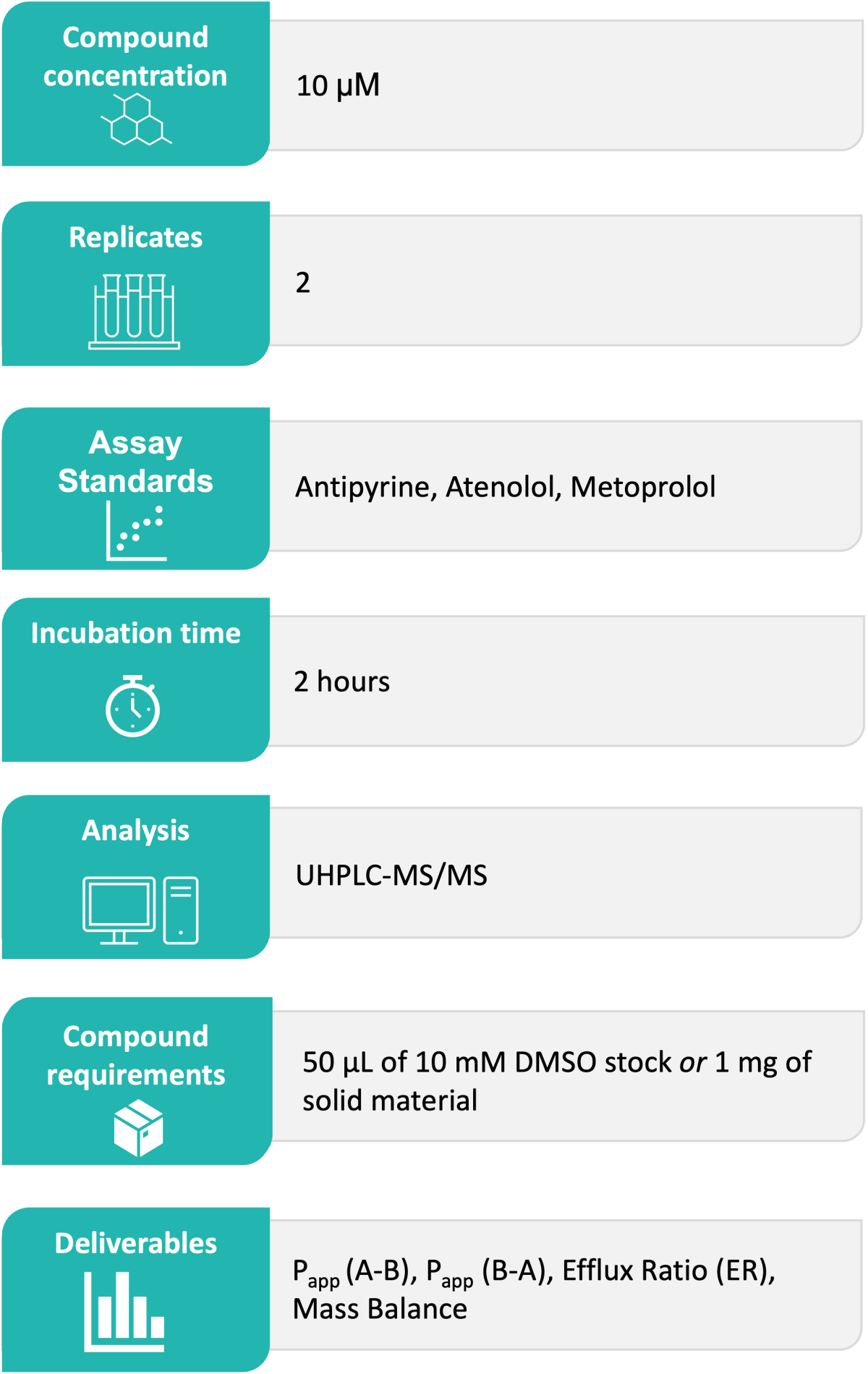
Compound is dosed on one side of the MDCK cell barrier, either apical or basal. The plate is then incubated for 120 minutes at 37 °C. A sample is taken from both sides of the MDCK cell monolayer, and levels of compound are quantified via Ultra-High Performance Liquid Chromatography (UHPLC)-Mass Spectrometry (MS) analysis.
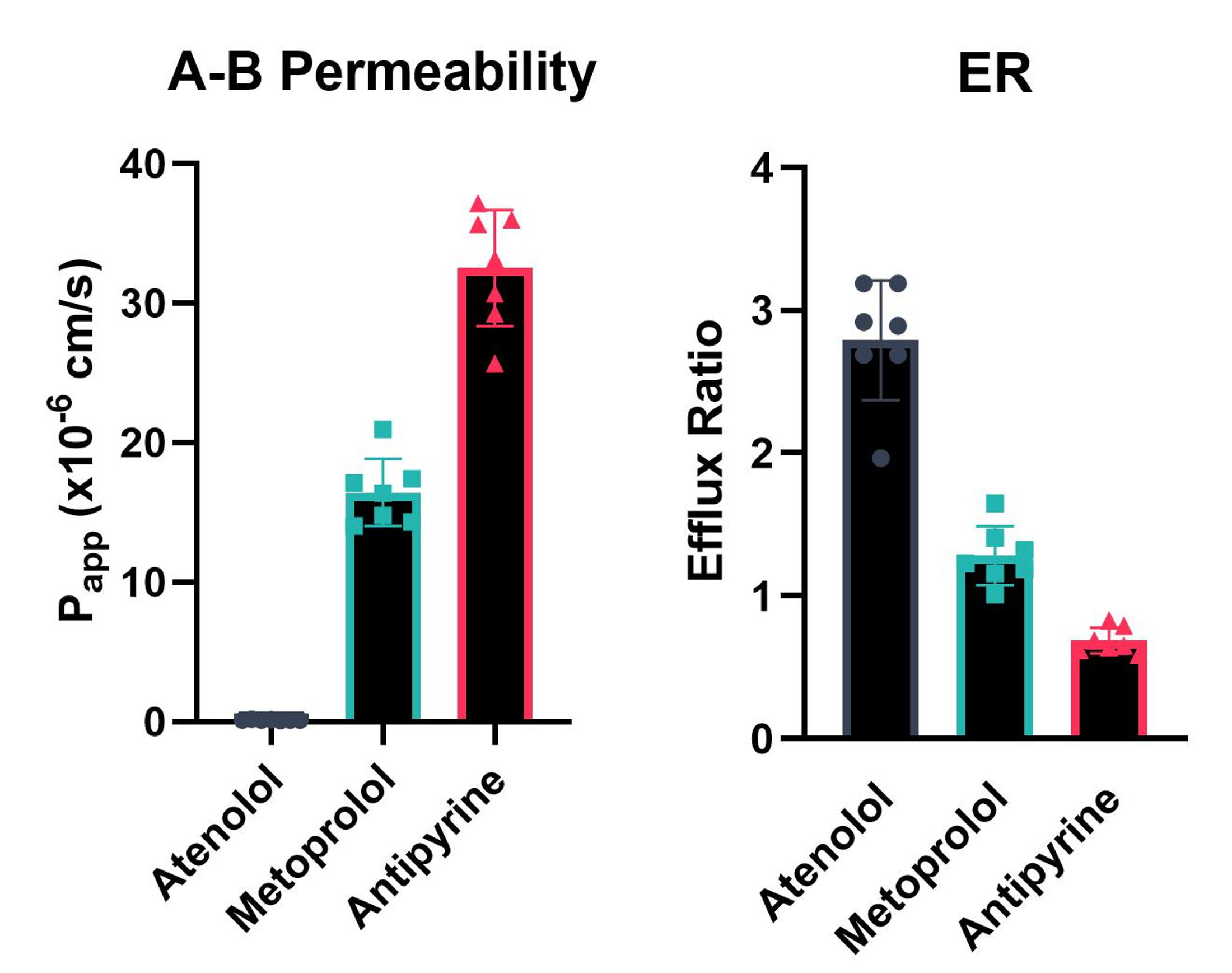
Prior to incubation, the integrity of the MDCK cell monolayer is determined via Trans-Epithelial Electrical Resistance (TEER) measurement. Following incubation, the integrity of the MDCK cell monolayer is confirmed via a Lucifer Yellow Paracellular Permeability assay. Example data is shown in Figure 1.
Data Analysis:
Supernatants from both apical and basal compartments are analysed using Waters Acquity UPLC TQ-S, TQ-S-micro or TQ-XS instruments. Triple quadrupole mass spectrometers operated in multiple reaction monitoring (MRM) mode, provide accurate quantification with excellent sensitivity, selectivity and reproducibility.
Deliverables:
The results are reported in Excel file format as Papp(A-B, 10-6 cm *s-1), Papp(B-A, 10-6 cm * s-1), efflux ratio (ER) and compound mass balance (%). Any relevant comments about compound stability/solubility/binding are also included in the report.
Turnaround time for MDCK Permeability Assay is available on request.
*Other options available on request
Start your next project with Domainex
Contact one of our experts today