- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
hERG Binding Assay
Cardiac arrhythmias are common adverse side effects of drug therapies.
This is often related to a compound’s ability to inhibit the human Ether-a-go-go-Related Gene (hERG) potassium channel, which is crucial for cardiac repolarisation. Inhibition of hERG is known to lead to QT interval prolongation which can result in ventricular arrhythmia and sudden death.
Domainex’s hERG binding assay enables identification of compounds that interact with the hERG channel, supporting the evaluation of potential cardiac toxicity risks. The assay employs the Predictor™ hERG Fluorescence Polarization (FP) method—a cost-effective, relatively high-throughput approach that facilitates screening and prioritization of larger compound sets. Data generated using this technique have demonstrated strong correlation with patch-clamp electrophysiology results (Piper et al., 2008).
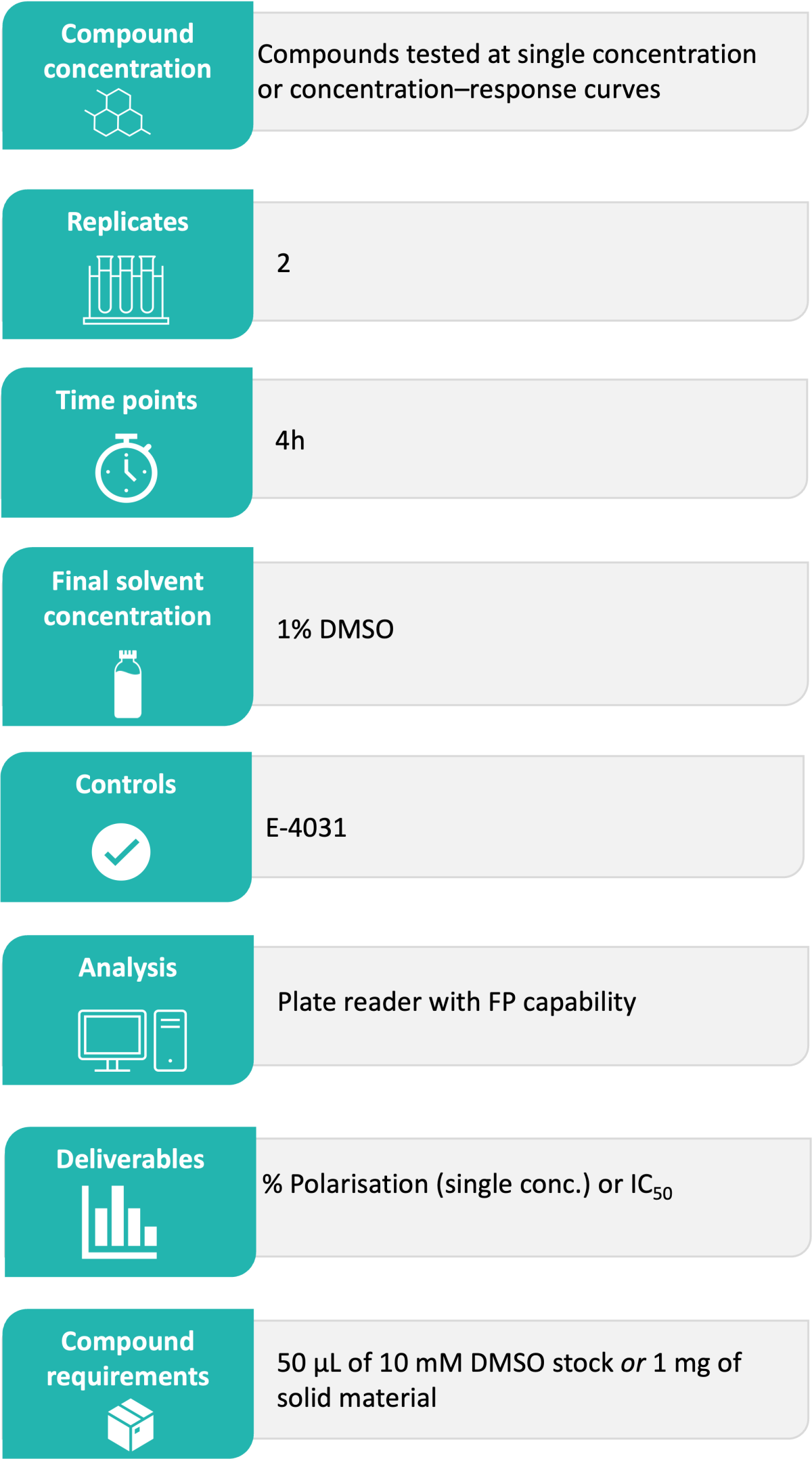
Domainex’s Standard Experimental Procedure:
Domainex’s hERG binding assay is available in two different formats, with compounds being tested either at a single concentration or by generating concentration-response curves (CRC).
Test compounds are incubated with hERG membranes and a tracer for 4 hours at room temperature. Fluorescence Polarisation is determined using CLARIOstar® Plus plate reader.
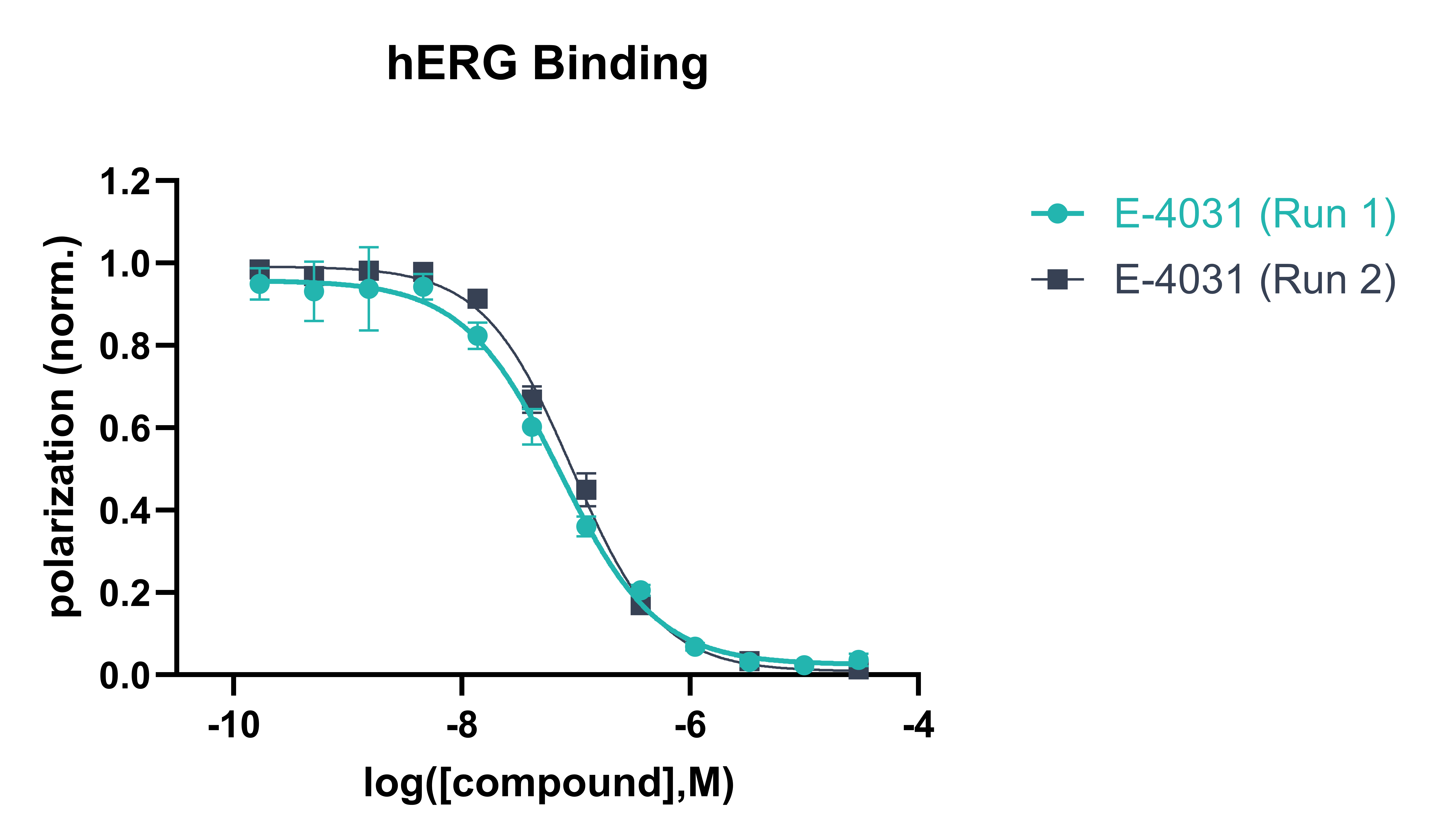
Data Analysis:
Polarisation values are background-corrected and normalised to high and low hERG binding controls.
For the CRC approach normalised polarisation values are plotted against compound concentration (log scale) to determine IC50 values.
Deliverables:
The results are reported in Excel file format as % polarisation (for single concentration) or IC50 values (for CRCs). Any relevant comments or observations made are also included in the report.
Typical turnaround time from receipt of test compounds to release of data is ten working days or less.
Start your next project with Domainex
Contact one of our experts today