- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Tankyrase: Inhibitors for the treatment of solid tumours
Novel, orally active drug candidates for Wnt-dependent tumours
Domainex employed its LeadBuilder virtual screening technology to identify tankyrase inhibitors from which pre-clinical development candidates emerged.

Challenge
Tankyrase is a member of the PARP family of enzymes and is a novel target involved in several pathways, including Wnt signalling. Therefore, Tankyrase inhibitors have potential as treatments for Wnt-dependent tumours, specifically colorectal cancers, of which around 60% are driven by aberrant Wnt signalling.1
Tankyrase activity leads to the “PARsylation” of Axin thereby targeting it for proteosomal degradation. So, inhibition of tankyrase leads to the stabilisation of Axin, making it available to carry out its role in the “destruction complex”. This leads to decreased levels of (nuclear) β-catenin.
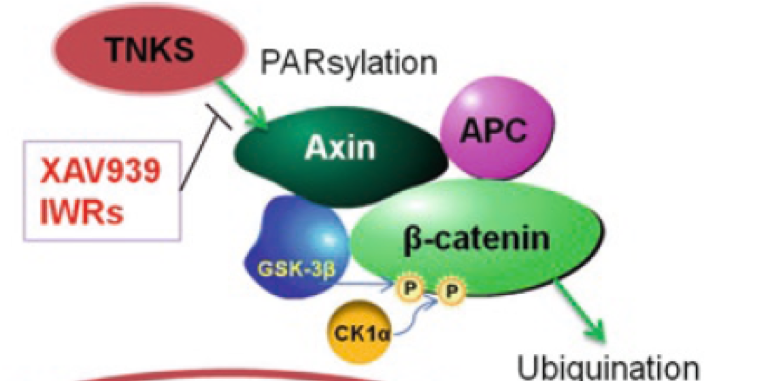
This target offers a clear unmet medical need with compelling biology and a “path-to-clinic”. Prior to starting this project there were no valid chemical starting points (“hits”), so the aim was to identify and develop a series of viable hits.
Solution
Hit identification
Domainex employed its LeadBuilder virtual screening technology to rapidly identify tankyrase inhibitor “hits”. The LeadBuilder approach significantly reduces the number of compounds that need to be screened in a ‘wet’ assay, and also accelerates the hit-to-candidate process by utilising a highly curated library containing chemically developable compounds with desirable physical properties.
Using this technology, around 1000 virtual hits were identified in just three weeks by one of our computational chemists. These were then screened in a tankyrase enzyme assay, and 59 hits were identified with >75% inhibition against tankyrase at 10μM or less.
Hit to candidate
With a number of attractive starting points in hand, the Domainex medicinal and computational chemists set about optimisation of these compounds. One of the classes that was followed up was a series of isoquinolones. Given the good physicochemical and ADMET properties of this series, and guided by X-ray crystal structures of several members of the series bound within the tankyrase enzyme active site, it proved possible to quickly develop this into a potent and selective lead series with excellent cellular activity (Figure 3).2
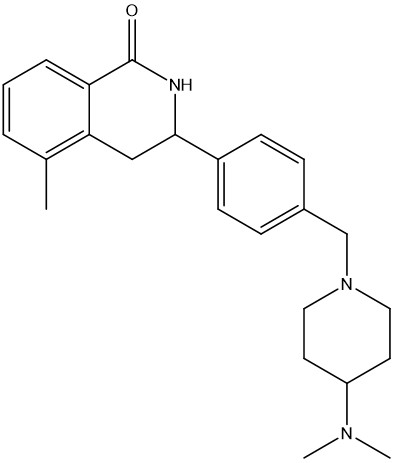
| Tankyrase IC50 | 13 nM |
| WNT-luc reporter IC50 | 61 nM |
| Cell inhibition SF50 (APCnull) | 80 nM |
| MW | 377.5 |
| LE | 0.38 |
Figure 3: Example of an isoquinolone tankyrase inhibitor
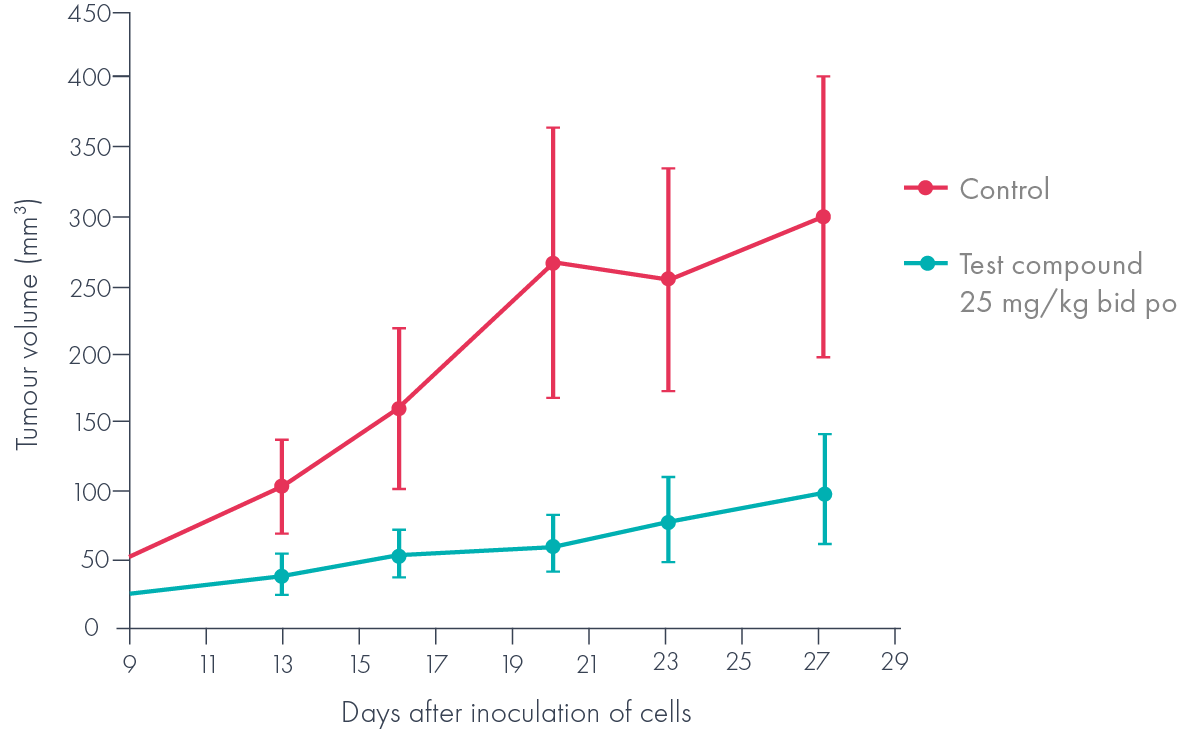
The low nM suppression of Wnt signalling combined with promising in vivo pharmacokinetics in mice (F>50%, t½ >1h) made these compounds attractive candidates for an in vivo proof of concept experiment, where it was shown that they inhibited the growth of a Wnt-dependent (APC null) tumour xenograft (Figure 4).
Further optimisation of this series afforded a candidate drug, which arose through the design and synthesis of less than 400 compounds starting from the original LeadBuilder hit. Moreover, another of the LeadBuilder hit compounds was also developed into a structurally distinct second series that yielded a back-up candidate.
Conclusions
Domainex's LeadBuilder platform rapidly identified multiple hit series with affinities of 0.1–10µM. This provided a cost-effective method to access a number of chemical leads that could be quickly optimised using SBDD to provide a candidate drug and a chemically differentiated back-up series. Both series were taken into preclinical development, and our client, the ICR, was able to licence the programme to Merck Serono.
Some of this work has been published.2
Domainex Expertise
• Integrated Drug Discovery • Virtual Screening via LeadBuilder • Hit Identification • Structure-based Drug Design (SBDD)
• Medicinal and Synthetic Chemistry • Lead Optimisation • DMPK and ADME • Oncology
References
- APC mutations occur early during colorectal tumorigenesis. Steven M. Powell, Nathan Zilz, Yasmin Beazer-Barclay, Tracy M. Bryan, Stanley R. Hamilton, Stephen N. Thibodeau, Bert Vogelstein and Kenneth W. Kinzler. Nature, 1992, 359, 235–237
- Design and discovery of 3-aryl-5-substitutedisoquinolin-1-ones as potent tankyrase inhibitors. Richard J. R. Elliott, Ashley Jarvis, Mohan B. Rajasekaran, Malini Menon, Leandra Bowers, Ray Boffey, Melanie Bayford, Stuart Firth-Clark, Rebekah Key, Rehan Aqil, Stewart B. Kirton, Dan Niculescu-Duvaz, Laura Fish, Filipa Lopes, Robert McLeary, Ines Trindade, Elisenda Vendrell, Felix Munkonge, Rod Porter, Trevor Perrior, Caroline Springer, Antony W. Oliver, Laurence H. Pearl, Alan Ashworth and Christopher J. Lord. Med Chem Comm, 2015, 6, 1687-1692
Start your next project with Domainex
Contact one of our experts today